Method for extracting and separating uranyl ions from aqueous phase
A technology for separating uranyl ions and uranyl ions, which is applied in the field of nuclear fuel cycle, can solve the problems of not many, the extraction rate of uranyl ions is not very high, and achieves the effects of good application prospects, improved extraction capacity, and wide adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1. Extraction of UO from neutral aqueous phase containing uranyl ions 2 2+
[0018] When carrying out the uranyl ion extraction experiment, take 0.5 ml of ionic liquid containing a certain amount of CMPO (purchased from STREM, USA, product number: 15-3500) and 1.0 ml of an aqueous phase containing a certain amount of uranyl ions and other ions or compounds, Mix well, then vibrate for 30 minutes, after centrifugation and phase separation, take the upper aqueous phase to analyze the concentration of uranyl ions (Arsenazo III method). than D.
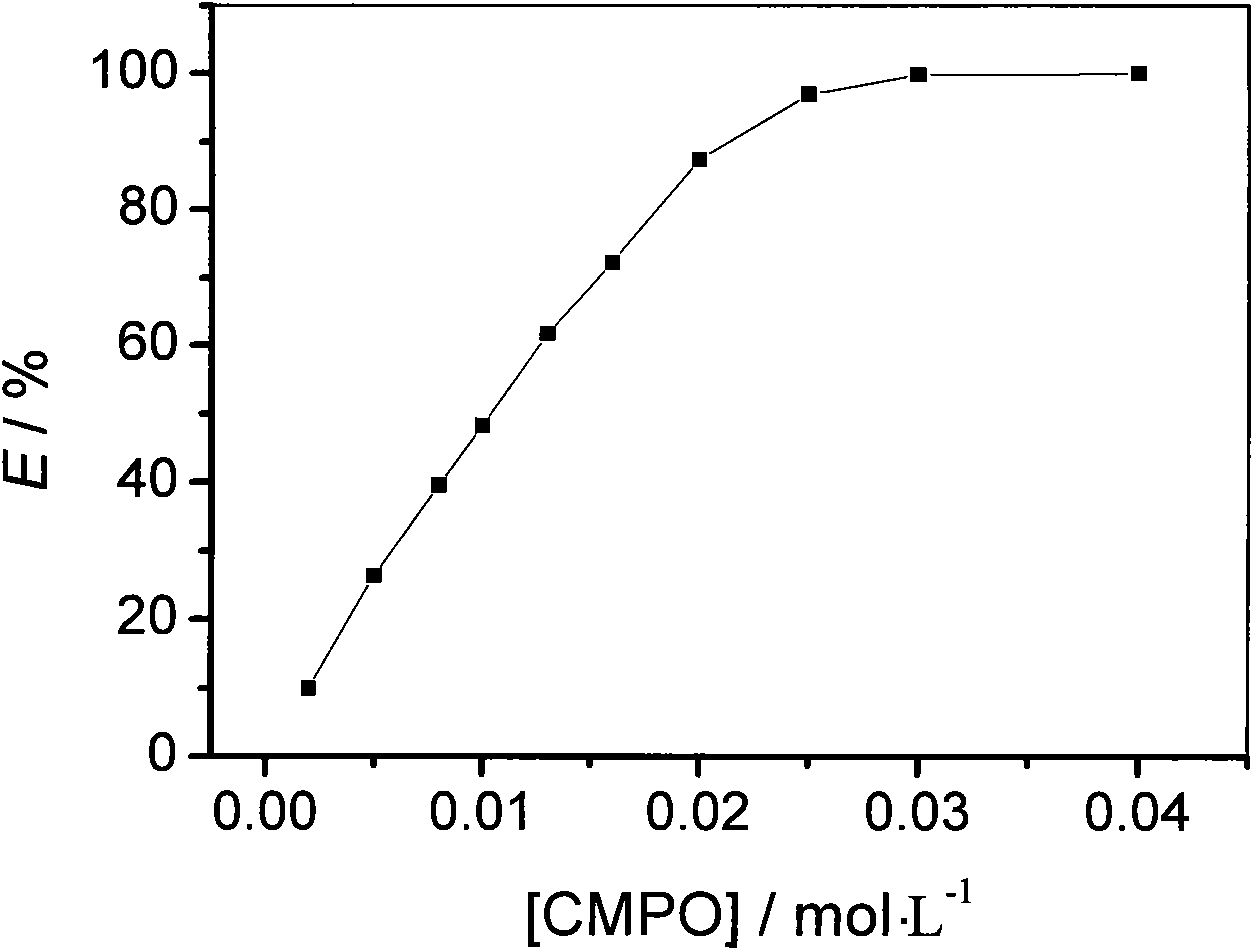
[0019] C containing different concentrations of CMPO 2 mimNTf 2 For uranyl ions ([UO 2 2+ ]=0.01mol / L) in the aqueous solution of uranyl ion extraction, the results are shown in Table 1.
[0020] Table 1
[0021]
[0022] With the uranyl ion extraction rate (E / %) and CMPO concentration plotting in table 1, the results are shown in figure 1 .
[0023] Depend on figure 1 It can be seen that under neutral conditio...
Embodiment 2
[0024] Example 2, Extraction of UO from the acidic aqueous phase containing uranyl ions 2 2+
[0025] The aqueous phase containing uranyl ions used in this example is acidic and does not contain other interfering ions.
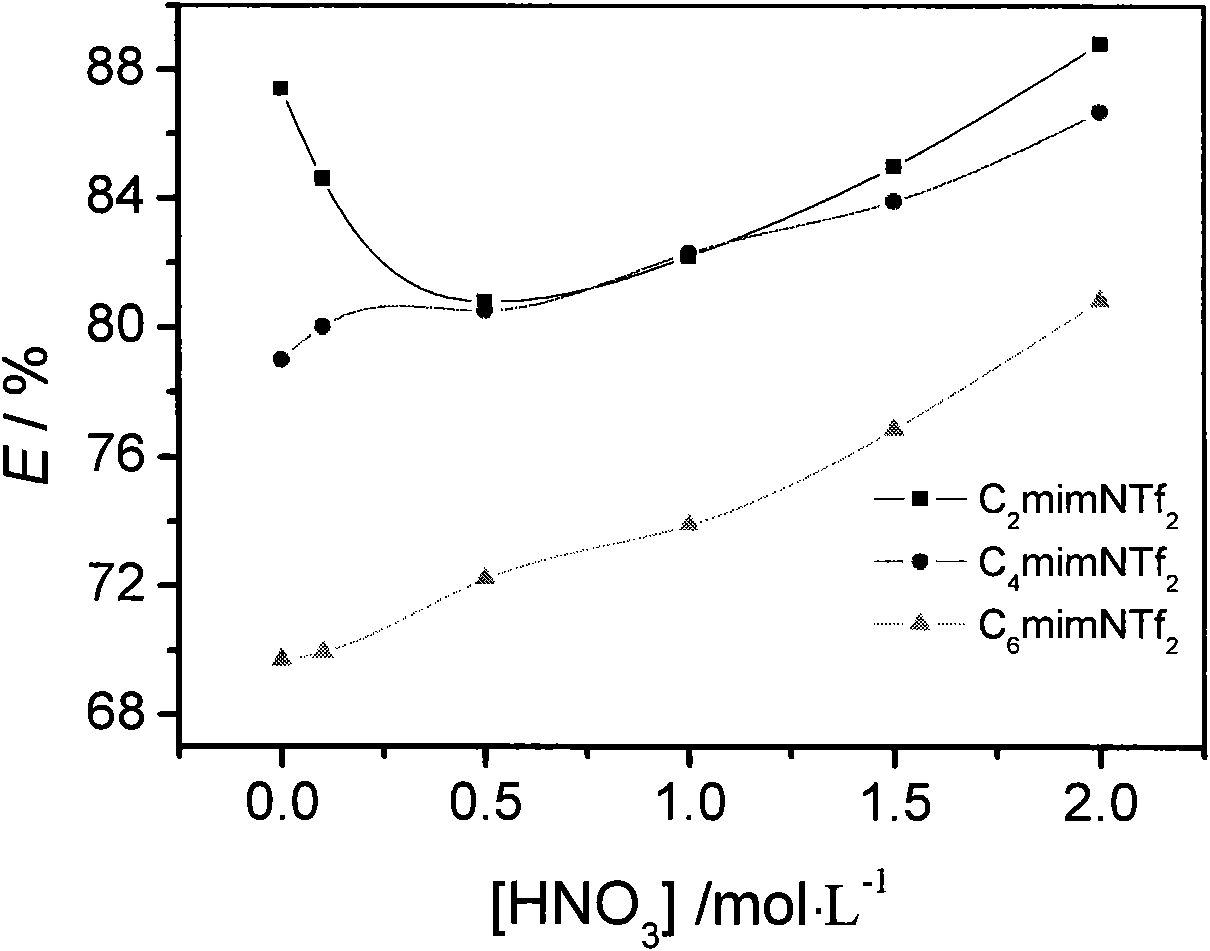
[0026] Using C containing CMPO concentration of 0.020mol / L n mimNTf 2 Effect of ionic liquids on uranyl ions ([UO 2 2+ ]=0.005mol / L, and the concentration of nitric acid is 0-3mol / L) for extraction, and the extraction rates are all substantially greater than 70%. The results are shown in Table 2.
[0027] Table 2
[0028]
[0029] With the uranyl ion extraction rate (E / %) in table 2 and the concentration of nitric acid in the aqueous phase, the results are shown in figure 2 .
[0030] Depend on figure 2 It can be seen that when different ionic liquids containing 0.02mol / L CMPO extract 0.005mol / L uranyl ions, the extraction rate changes with the concentration of nitric acid in the aqueous phase. Specifically, for C 4 mimNTf 2 、C 6 mimNTf 2 s...
Embodiment 3
[0032] Example 3, Extraction of UO from the aqueous phase containing uranyl ions and interfering ions such as sodium and potassium 2 2+
[0033] With 0.02mol / L CMPO / C 4 mimNTf 2 Extract 0.005mol / L UO 2 2+ system as an example, when the water phase contains 0.05mol / L~0.5mol / L of inorganic salt NaNO 3 or KNO 3 , the extraction rate of 0.005mol / L uranyl ions in the system is between 72-76% (see Table 3 for details), which is slightly lower than the extraction rate (79%) of the system for uranyl ions in the absence of inorganic salts, indicating that NaNO 3 or KNO 3 It has little effect on the extraction of uranyl ions from the system.
[0034] table 3
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com