Stroke recovery
A hydroxylamine derivative, hydroxyl technology, applied in the field of hydroxylamine derivatives for the treatment of stroke, can solve problems such as limited therapeutic application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0062] The present invention relates to a method of treating stroke comprising administering an effective amount of one or more of certain hydroxylamine derivatives to a patient in need thereof, i.e. a patient diagnosed as having suffered from stroke, exhibiting stroke-related Patients with relevant symptoms or surrogate indicators and / or suspected stroke. Hydroxylamine derivatives useful in the practice of this method include those previously described in the following patent documents:
U.S. Patent No. 5,147,879; U.S. Patent No. 6,143,741; U.S. Patent No. 6,653,326;
U.S. Patent No. 6,649,628; U.S. Patent No. 6,384,029; U.S. Patent No. 5,328,906;
U.S. Patent No. 5,296,606; U.S. Patent No. 5,919,796; U.S. Patent No. 6,002,002
US Patent No. 6,180,787; US Patent No. 6,384,029; and US Patent Publication No. 2005 / 0043295, all of which are incorporated herein by reference.
[0063] In one embodiment, the method comprises the step of administering N-[2-hydroxy-3-(1-piperidinyl...
Embodiment 1
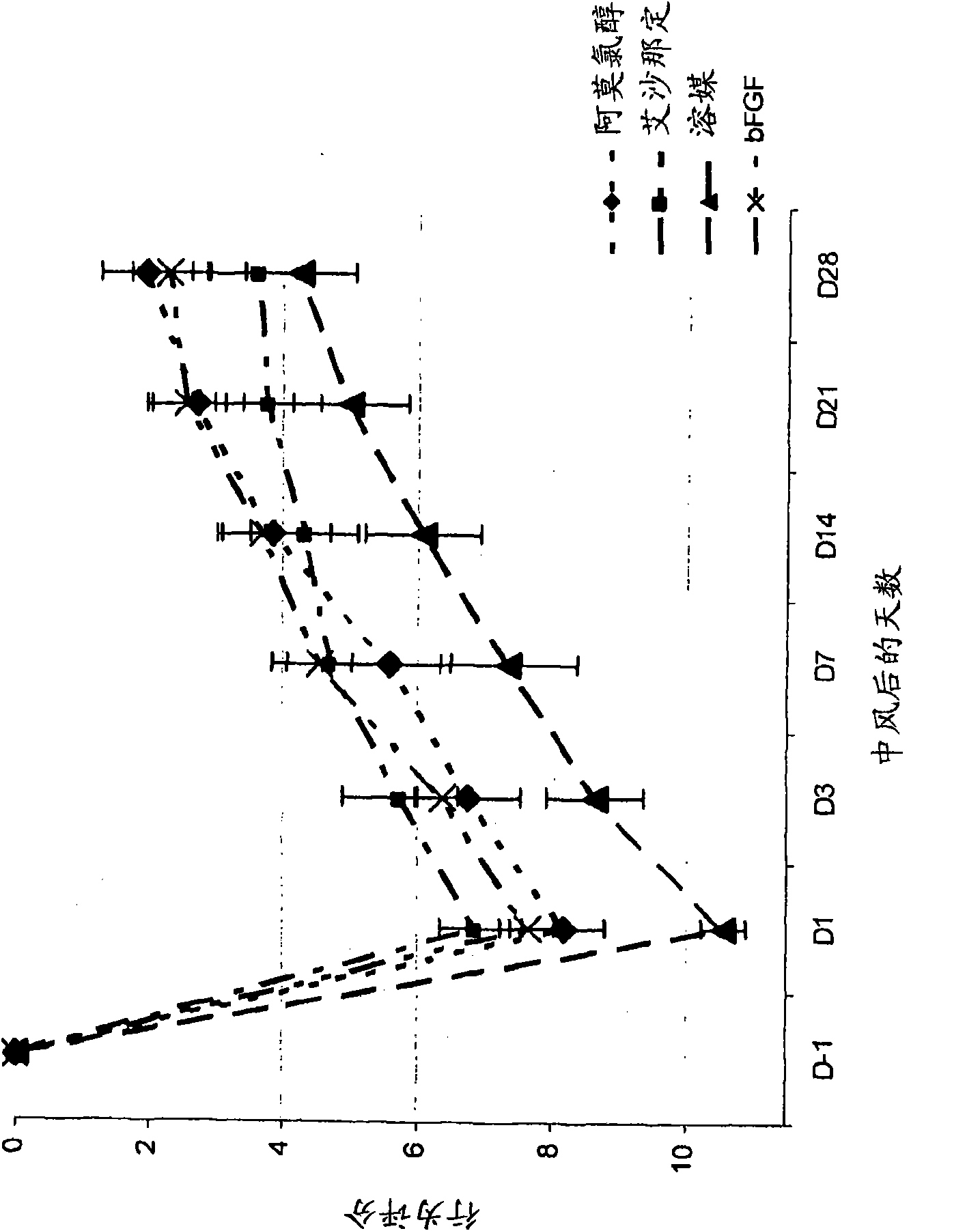
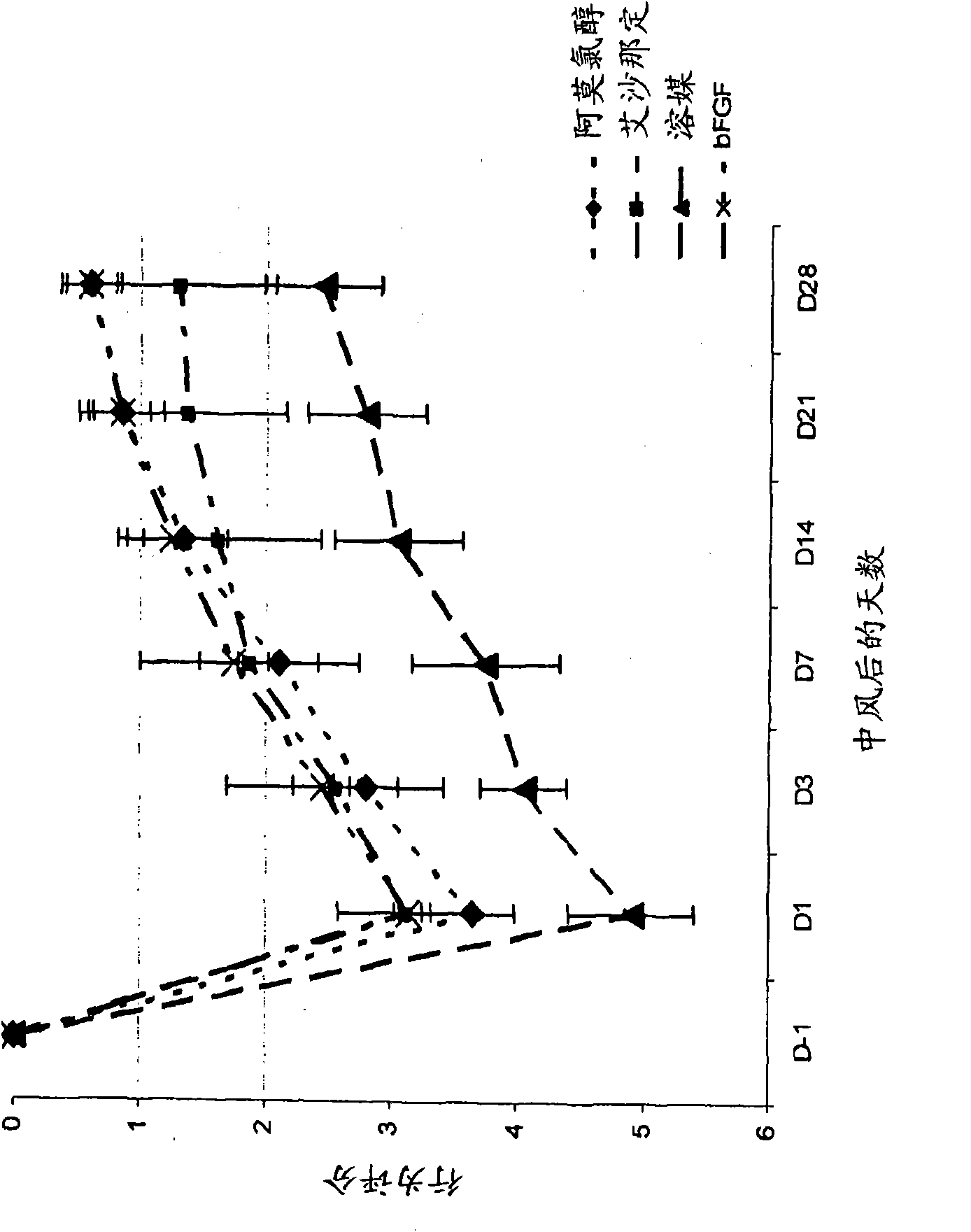
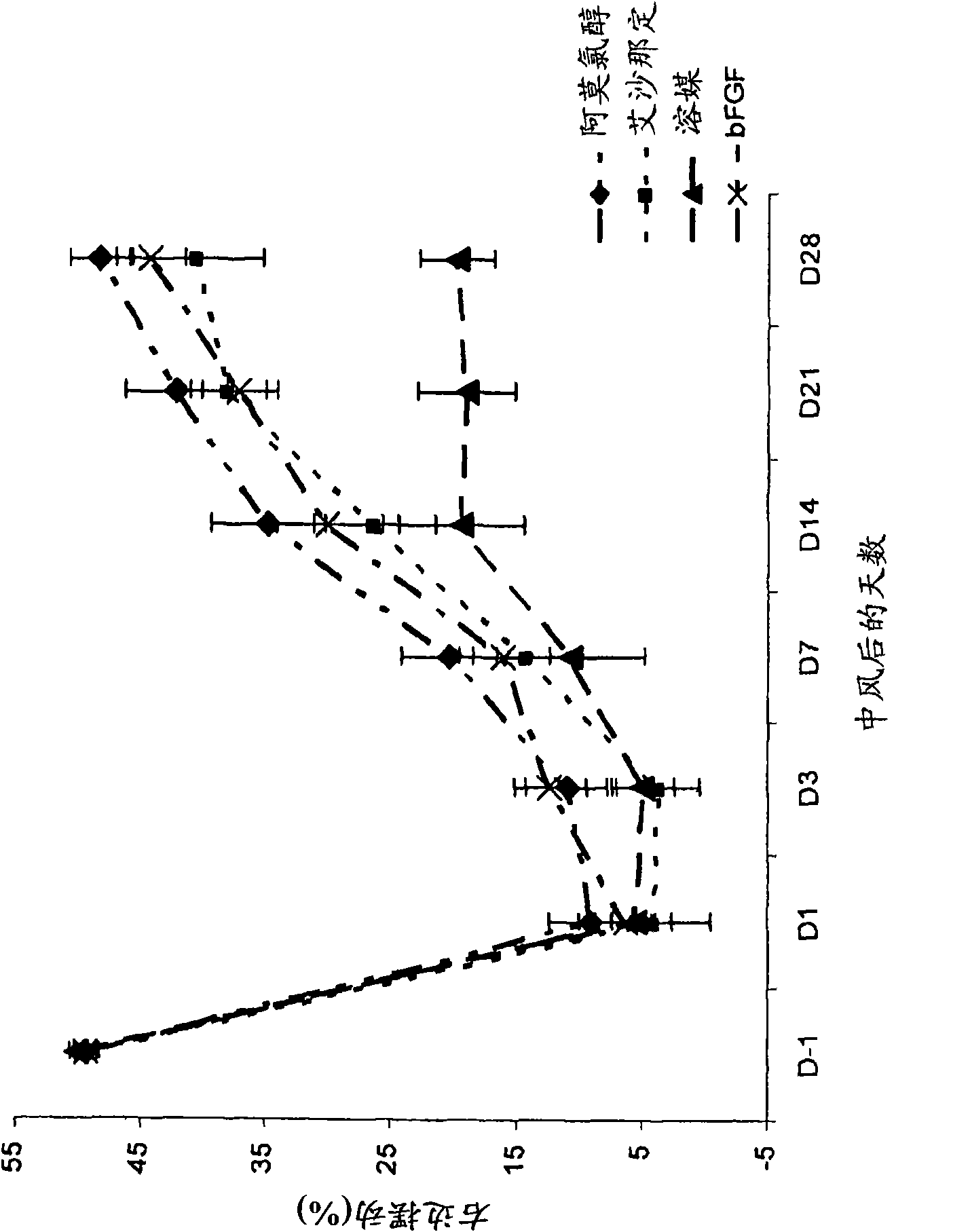
[0178] The aim of this study was to evaluate the efficacy of amoclorol and exenadine in enhancing neurorehabilitation in a rat model of permanent middle cerebral artery occlusion (MCAO). Permanent MCAO is well accepted and considered the standard animal model for studying the clinical aspects of stroke. (Stroke.1999; 30:2752-2758.)
[0179] Forty male Sprague Dawley rats, each weighing 300-400 g, were caged and manipulated for behavioral assessment for 7 days prior to surgery for acclimatization purposes. These rats were placed under anesthesia according to the modified Tamura model Surgery was performed to create a partial cerebral infarction by permanently occluding the proximal right middle cerebral artery (MCA). Briefly, using the N 2 O:O 2 Rats were anesthetized with 2-3% halothane in a (2:1) mixture and treated with N 2 O:O 2 Anesthesia was maintained with 1-1.5% halothane in a (2:1) mixture. The temporalis muscle is divided in half and reentranted through the inci...
Embodiment 2
Example 2. Functional rehabilitation after MCA occlusion administered with amoclorol in rats - dose study.
[0193] Fifty male Sprague Dawley rats, each weighing 300-400 g, were operated under anesthesia as described in Example 1 herein to cause MCA occlusion, and the rats were divided into 5 groups of 10 animals each. Each group was given p.o. amochlorohydrin, starting one day after occlusion, at 25 mg / kg / d, 50 mg / kg / d, 100 mg / kg / d or 200 mg / kg / d, once a day for 35 days. One group was the control group, given vehicle only.
[0194] Animals were evaluated pre-surgery (day-1) and then every 7 days (7, 14, 21, and 28 days) after surgery by the forelimb and hindlimb placement test and the body swing test, as described in Example 1 herein. out rating.
[0195] On day 35 after MCAO, rats in the control and highest dose groups were sacrificed and their brains were evaluated as in Example 1. Rats in other groups were sacrificed and their brains excised and flash frozen for further ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com