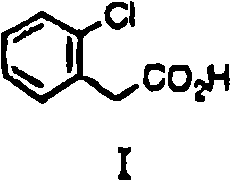Process for producing intermediate of asenapine synthesis
A compound and reaction technology, applied in the preparation of organic compounds, carboxylate salts, carboxylic acid amides, etc., can solve the problems of complicated preparation and difficult use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0081] Hereinafter, although this invention is demonstrated concretely based on an Example, it cannot be overemphasized that this invention is not limited to these Examples.
[0082] [Example]
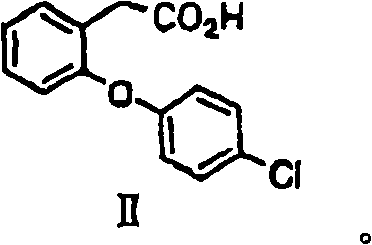
[0083] (1-a) Synthesis of 2-(2-(4-chlorophenoxy)phenyl)acetic acid [II]
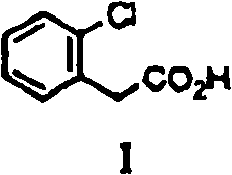
[0084] 2-Chlorophenylacetic acid [I] (1.0g, 5.9mmol), 4-chlorophenol (0.78g, 6.5mmol), cesium carbonate (3.8g, 11.7mmol), copper bromide (I) (42mg, 0.29 mmol) and diethylene glycol dimethyl ether (5 ml) were mixed and heated at 145°C for 8 hours. After cooling, water (15ml) was poured into the reactant, then concentrated hydrochloric acid (about 2g) was added to make it acidic, and it was extracted with toluene (20ml×2). After the toluene layer is washed with water (20ml), saturated brine (20ml), dehydrate with anhydrous magnesium sulfate, remove magnesium sulfate by filtration then, obtain the toluene solution of [II] (the quantitative yield of using standard substance (standard product) is 59%). For use i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com