Polypeptide for preparing anti-PINK1 polyclonal antiserum and application thereof
A polyclonal antiserum, antiserum technology, applied in applications, antibodies, recombinant DNA technology, etc., can solve problems such as unclear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0046] In the following examples, unless otherwise specified, the methods used are conventional methods, and the reagents used can be obtained from commercial sources.
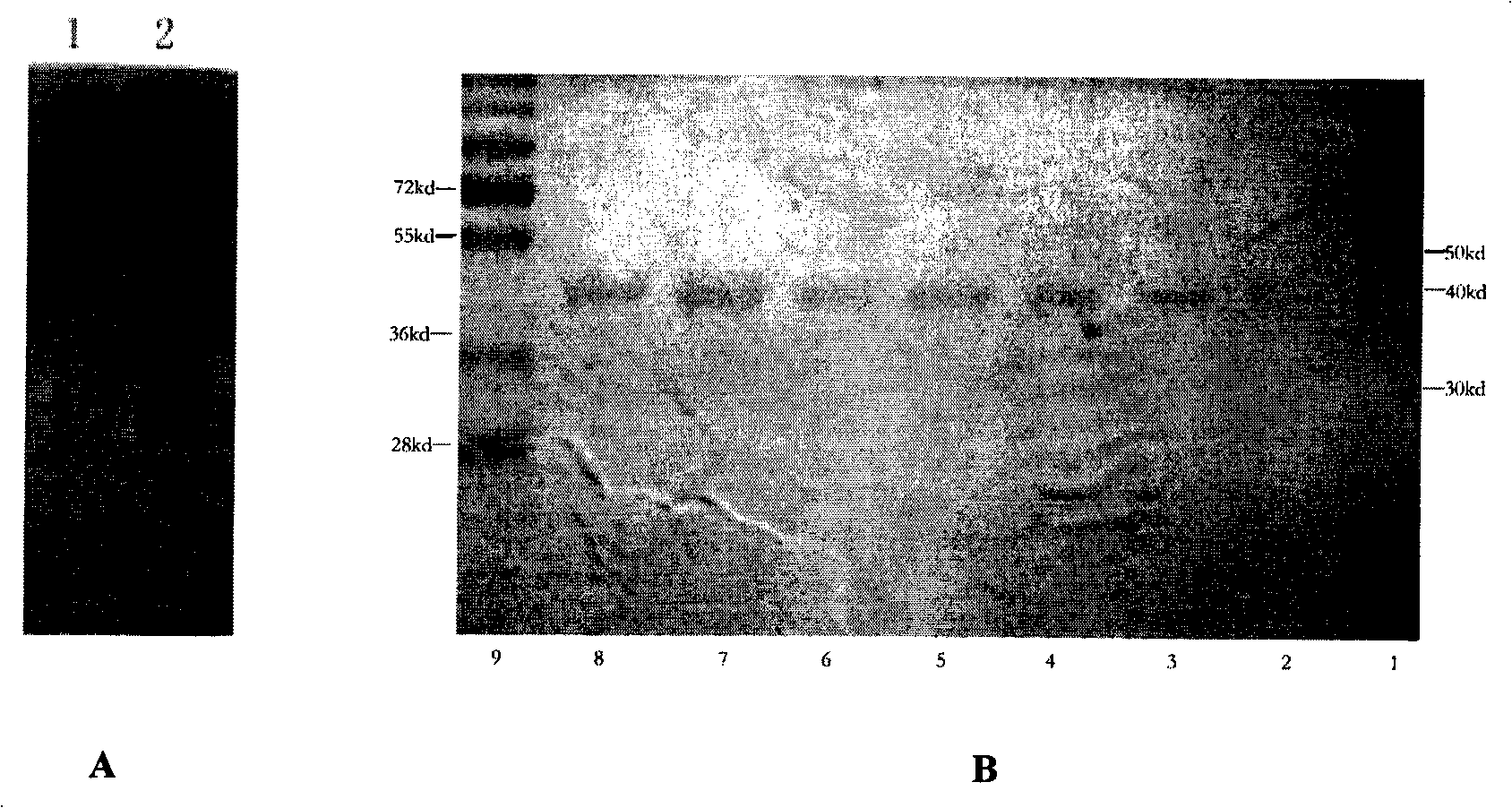
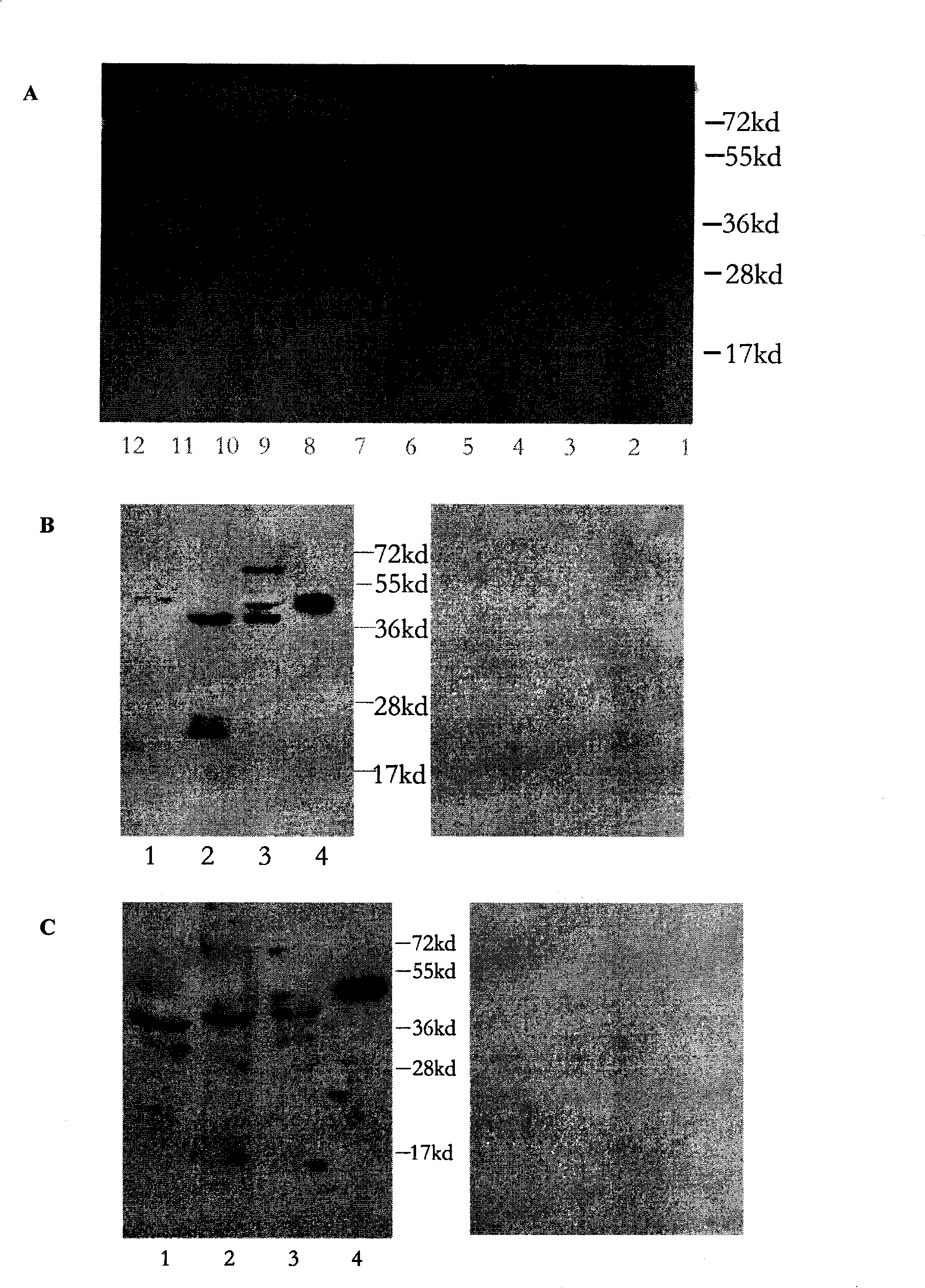
[0047] 1. Expression and purification of recombinant human PINK1 for animal immunization
[0048] The kinase domain of human PINK1 consists of 425 amino acids, and its amino acid sequence is shown in sequence 1 in the sequence listing. Total RNA was extracted from normal human embryonic brain, reverse-transcribed into cDNA, and PCR-amplified with primer 1 (5`CCC AAG TTT GTT GTG ACC G3`) and primer 2 (3`TGT AAT TTC CCA CTC CCGTA5`) encoding PINK1 The DNA encoding the kinase domain of human PINK1 obtained by PCR amplification was inserted into the pET-28a vector to form the recombinant plasmid pET-28a-PINK1. Transform pET-28a-PINK1 into BL21(DE3) competent cells, take a single colony and inoculate into 10mL 2×YTA-amp liquid medium (each liter contains 16g peptone, 10g yeast extract, 5g NaCl and 0.1g ampicillin)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com