Methods for analysing protein samples based on the identification of c-terminal peptides
A carboxy-terminal peptide and protein technology, applied in the field of data analysis of technology and mass spectrometry data, can solve the problem that peptides cannot be attributed and achieve the effect of reducing complexity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] Example 1: Separation of carboxy-terminal peptides
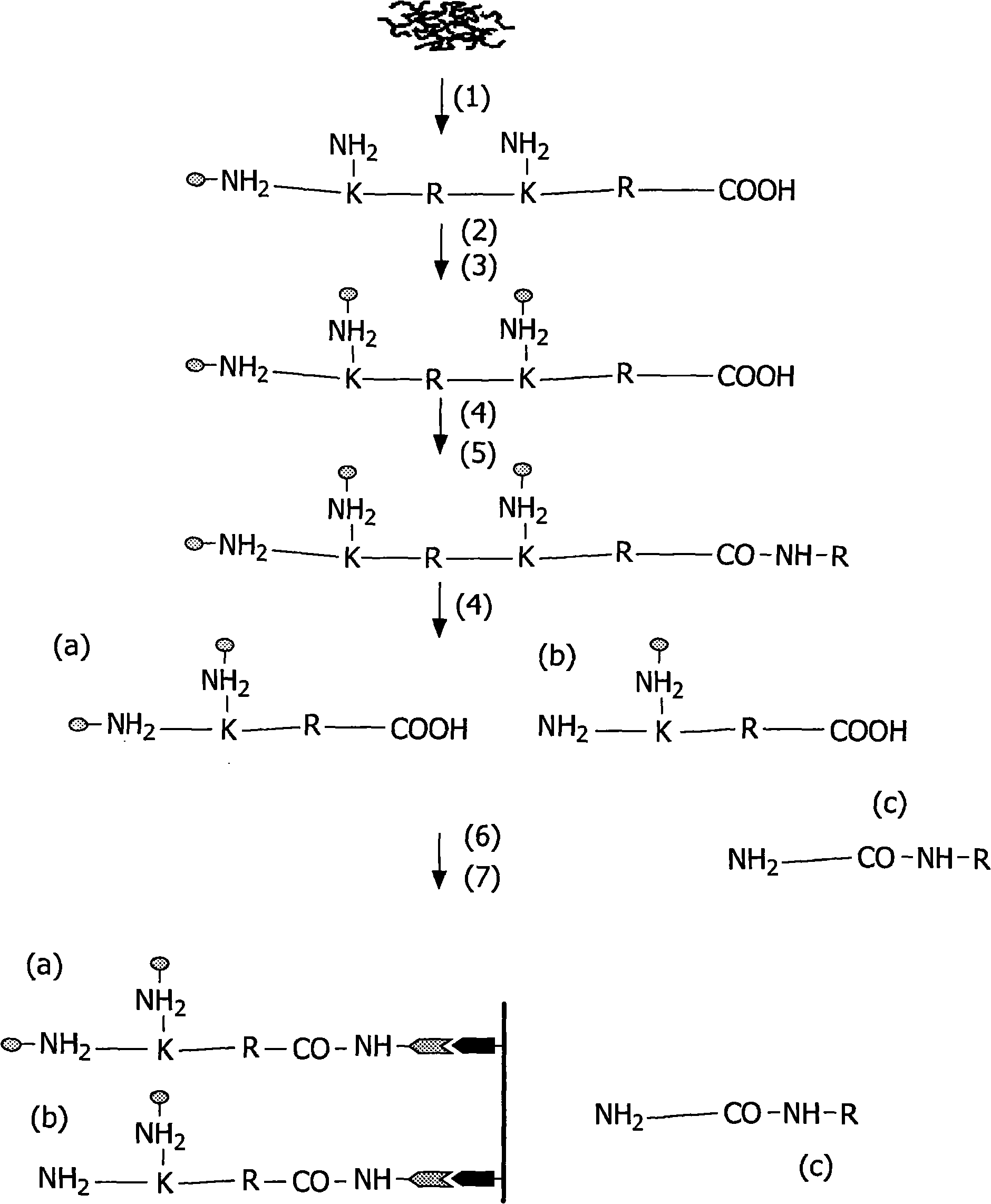
[0140] The flow chart for the isolation of carboxy-terminal peptides is at figure 1 in brief.
[0141] Isolate protein extracts from tissues using standard methods. The side chain of cysteine is alkylated and the amine at the amino terminus and the side chain of lysine are acetylated. In the next step, 1-ethyl-3-[3-dimethylamino-propyl]carbodiimide hydrochloride was added according to the method as shown in Grabarek and Gergely (1990) Anal Biochem. (EDC) or 1-ethyl-3(3-dimethyl-aminopropyl)-carbodiimide (EDAC) activates the free carboxyl group of the carboxy-terminal amino acid (and the reactivity on glutamate and aspartate carboxyl).
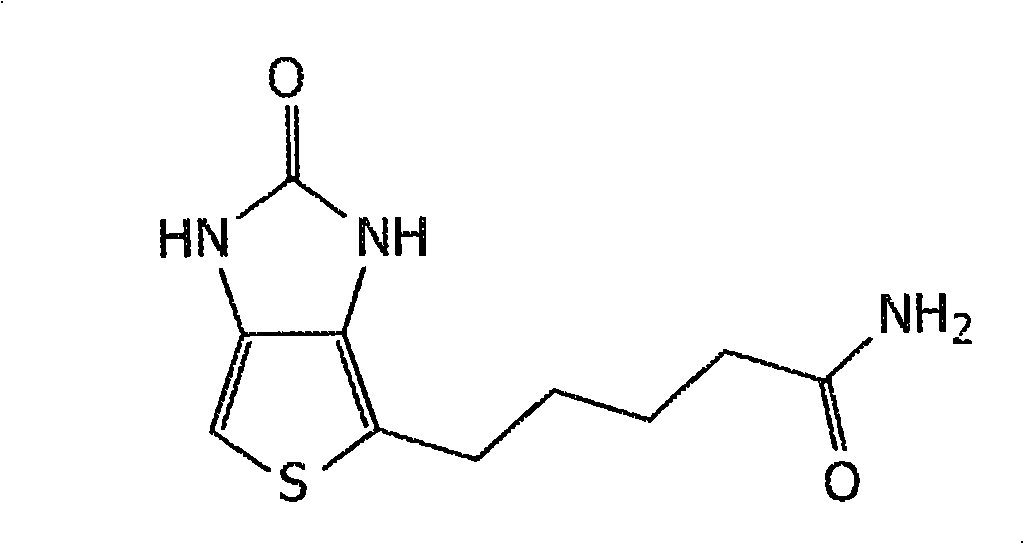
[0142] EDC and protein ( figure 2 The carboxyl group on molecule 1) in reacts to form an amine-reactive O-acylisourea intermediate. This intermediate can be combined with NH-R( figure 2 The amine reaction on molecule 2) in , produces a conjugate of these two molecules linked...
Embodiment 2
[0147] Example 2: Identification of peptides with similar masses
[0148] This example shows the need to supplement peptide quality data with additional parameters. From Prosite(ScanProsite on www.expasy.org / prosite), the sequence SFPNIGSL [SEQ ID NO: 1] of an 8-amino acid carboxy-terminal tryptic peptide of a human protein with potential clinical relevance, Exostosin 2, was selected.
[0149] The calculated average mass (833.94) of SEQ ID NO: 1 was used to identify Profound (prowl.rockefeller.edu) peptides with calculated masses in the range of theoretical 1 Da. This calculation was performed by performing an in silico trypsinization of human protein (see Table 1 ) which does not allow partial cleavage and does not select a large number of peptides which are carboxy-terminal.
[0150] Table 1. Masses and physicochemical parameters of carboxy-terminal peptides with relevant masses
[0151] 1
2
3
4
5
6
7
quality
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com