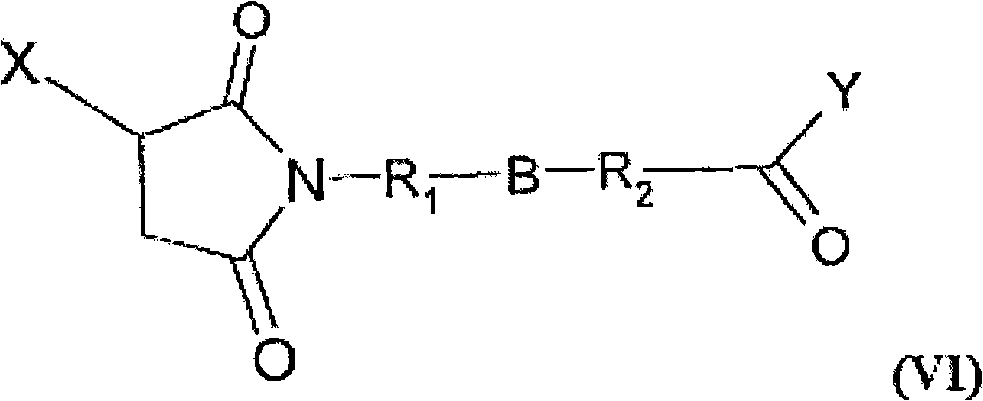Camptothecin-peptide conjugates and pharmaceutical compositions containing the same
A conjugate and camptothecin technology, applied in the field of new compounds, can solve problems such as insufficient intracellular delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
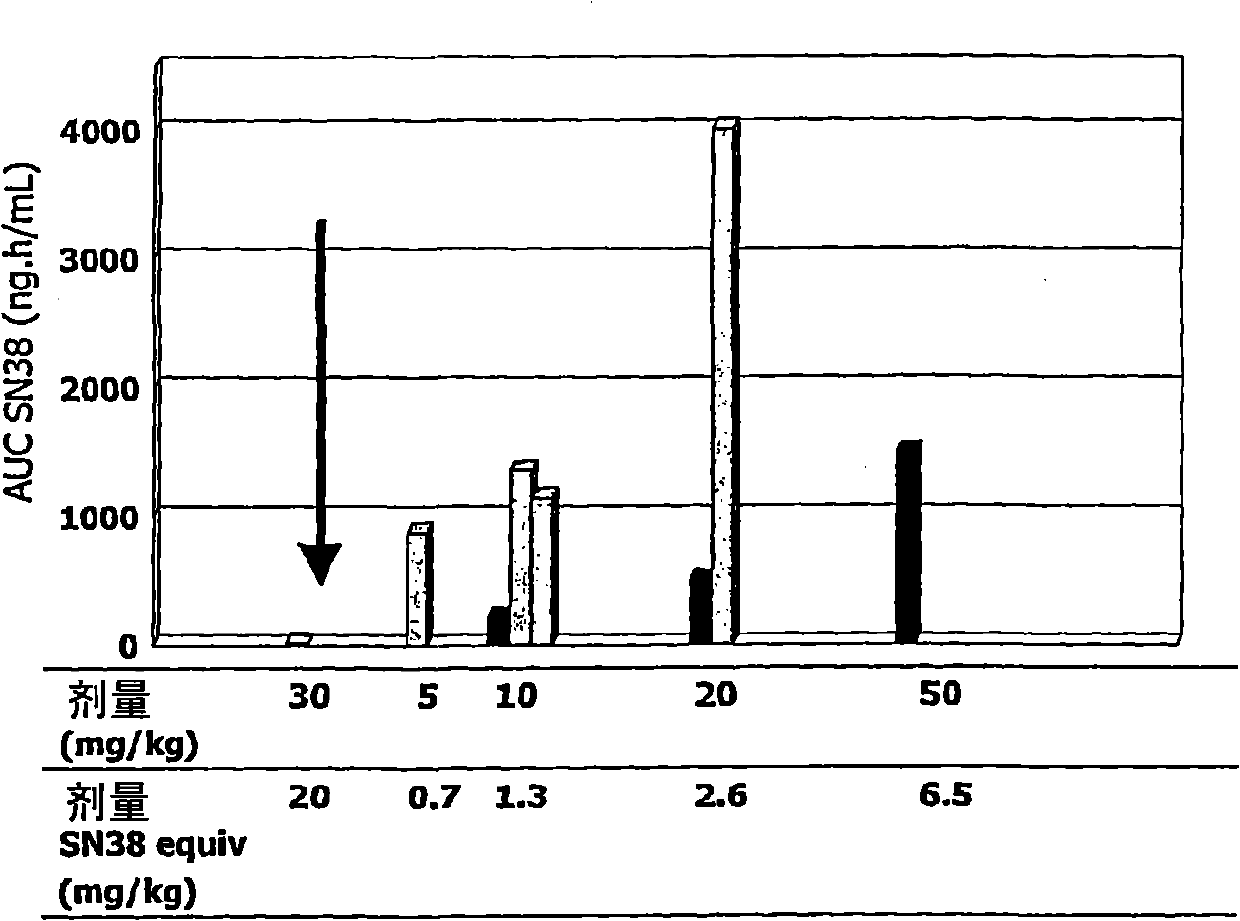
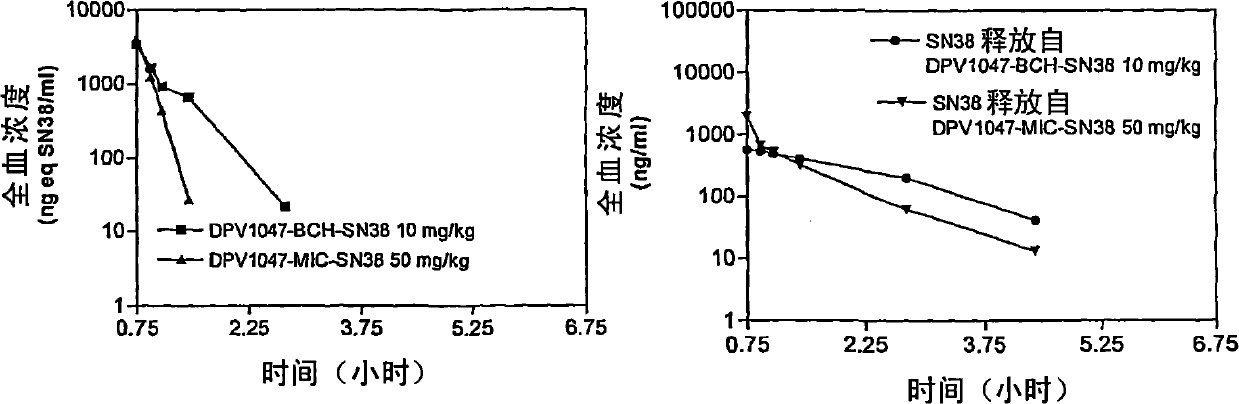
[0226] I. Materials and methods
[0227] I.1a Cell Penetrating Peptide (CPP)
[0228] DPV3: Arg Lys Lys Arg Arg Arg Glu Ser Arg Lys Lys Arg Arg Arg Glu Ser (SEQ ID NO.1)
[0229] DPV1047: Val Lys Arg Gly Leu Lys Leu Arg His Val Arg Pro Arg Val Thr Arg Met Asp Val (SEQ ID NO.8)
[0230] DPV15: Leu Arg Arg Glu Arg Gln Ser Arg Leu Arg Arg Glu Arg Gln Ser Arg (SEQ ID NO.10)
[0231] DPV15b: Gly Ala Tyr Asp Leu Arg Arg Arg Glu Arg Gln Ser Arg Leu Arg Arg Arg Arg Glu ArgGln Ser Arg (SEQ ID NO.11)
[0232] DPV7: Gly Lys Arg Lys Lys Lys Lys Gly Lys Leu Gly Lys Lys Lys Arg Asp Pro (SEQ ID NO.3)
[0233] Tat 48-60 : Gly Arg Lys Lys Arg Arg Gln Arg Arg Arg Pro Pro Gln (SEQ ID NO.44)
[0234] Penetrating peptide: Arg Gln Ile Lys Ile Trp Phe Gln Asn Arg Arg Met Lys Trp Lys Lys (SEQ ID NO.29)
[0235] DPV51 (D) : D-Lys D-Arg Gly D-Leu D-Lys D-Leu D-Arg D-His (SEQ ID NO.51)
[0236] DPV1047 (D) : Val Lys Arg Gly Leu Lys Leu Arg His Val Arg Pro Arg Val Thr Arg Met AspVal (SEQ ID ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com