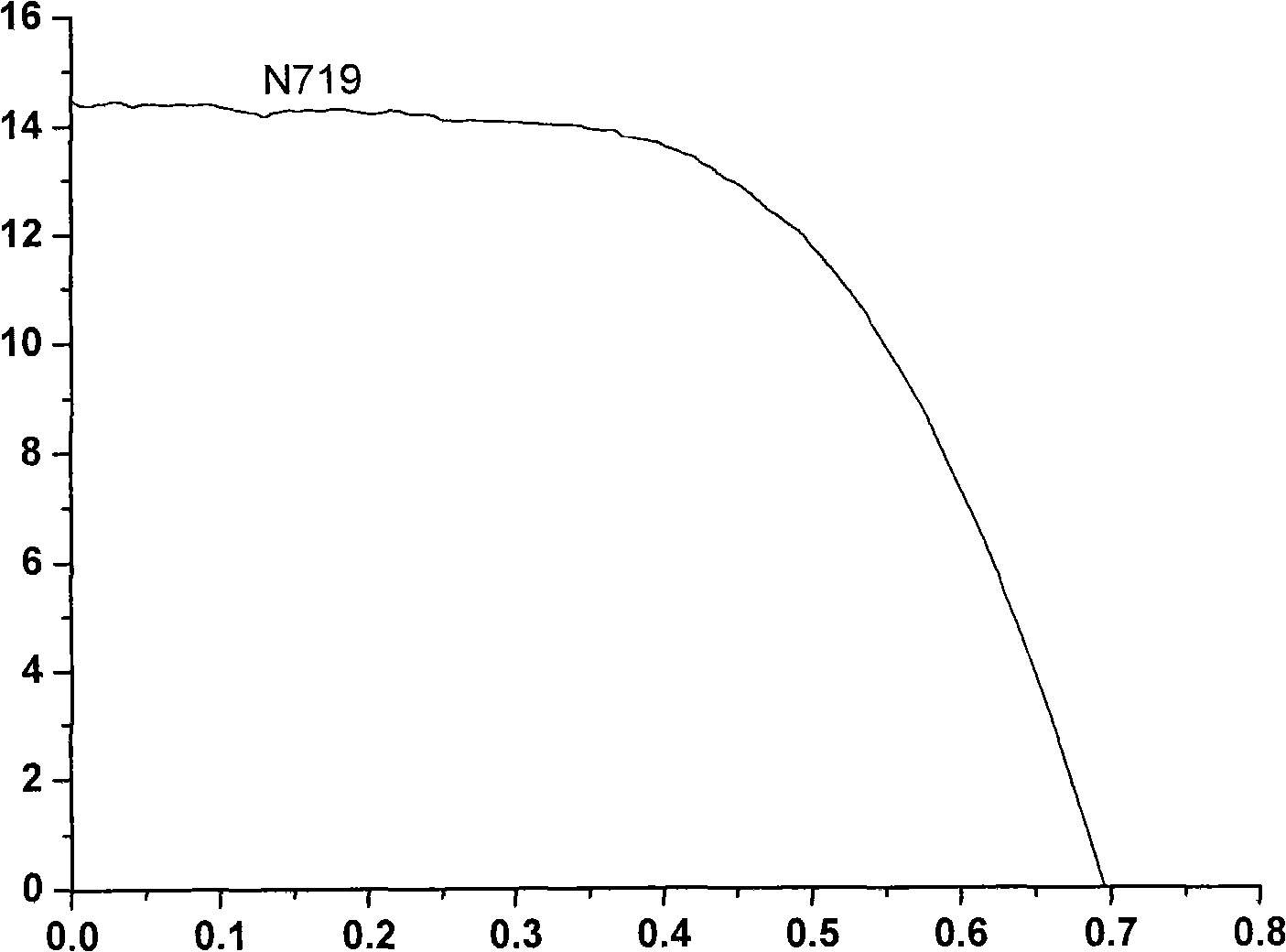
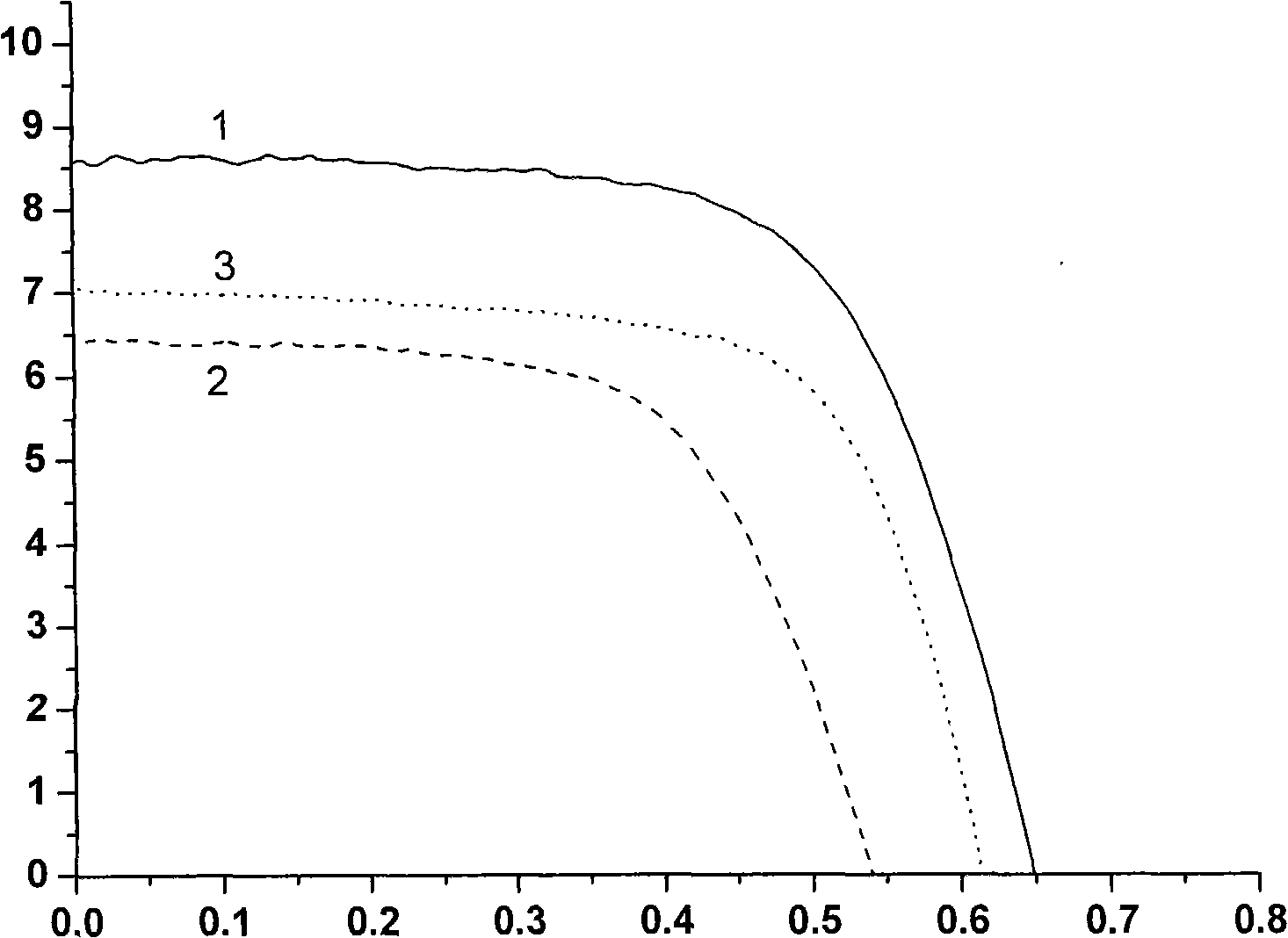
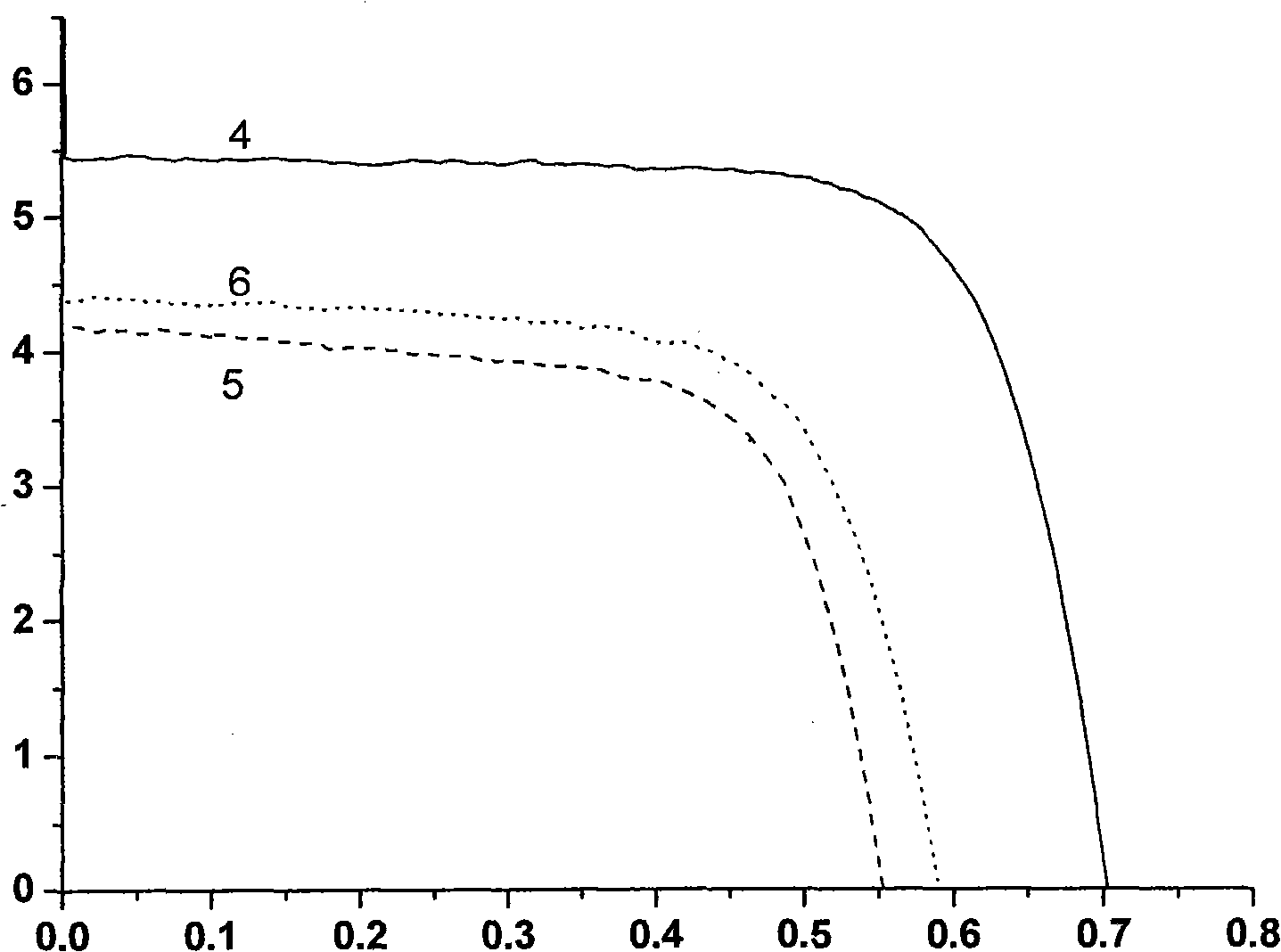
Ethylene naphthalene and paradiazine dye and application thereof to dye-sensitized solar cells.
A technology of solar cells and dye sensitization, which can be used in different fields and can solve the problems of high cost and limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Synthesis of 3-diphenylamine-8,9-dicarboxy-acenaphthopyrazine
[0030] (1) Synthesis of 5-bromoacenaphthylquinone:
[0031]
[0032] In a 500mL two-necked flask, add 20g (109.8mmol) of acenaphthylquinone and 25.0mL of liquid bromine (466.8mmol), start stirring, control the temperature in an oil bath at 6070°C, and reflux the reaction solution. After 2h, stop the reaction and add hydrogen sulfite Sodium aqueous solution until the reaction solution is colorless. After diluting with water, filter under reduced pressure, and wash with water for several times for beating until pH=7.0. The filter cake was recrystallized four times in glacial acetic acid to obtain 5-bromoacenaphthoquinone as brown-yellow needle-like crystals with a yield of 90%. NMR 1 H NMR (400MHz, DMSO-d 6 )δ8.39(d, 1H), 8.21(d, 1H), 8.15(d, 1H), 8.04(t, 1H), 7.96(d, 1H); HRMS-EI(70eV) m / z.
[0033] (2) Synthesis of 5-diphenylamine-acenaphthenequinone:
[0034]
[0035] The reaction is...
Embodiment 2
[0043] Example 2: Synthesis of 3-diphenylamine-8,9-dicarboxy-acenaphthopyrazine-acene
[0044] (1) Synthesis of 3-diphenylamine-8,9-dicyano-acenaphthopyrazine-acene
[0045]
[0046] Synthetic method is substantially the same as (3) in embodiment 1, nuclear magnetic 1 HNMR ((CDCl 3 )): δ7.12-7.16(m, 6H), 7.32(t, 4H), 7.46(d, 1H), 7.62(t, 1H), 7.86(d, 1H), 8.36(d, 1H), 8.42 (d, 1H), 8.60 (d, 2H).FAB MS: m / e 471 (M + ).
[0047] (2) Synthesis of 3-diphenylamine-8,9-dicarboxy-acenaphthopyrazine-acene
[0048]
[0049] Synthetic method is substantially the same as (4) in embodiment 1, nuclear magnetic 1 HNMR ((DMSO-d 6 )): δ7.09-7.14(m, 6H), 7.35(t, 4H), 7.44(d, 1H), 7.30(t, 1H), 7.80(d, 1H), 8.38-8.41(m, 4H) .MS API-ES: [M-H] + (508, M / Z).
Embodiment 3
[0050] Example 3: Synthesis of 3-diphenylamine-8-carboxy-acenaphthopyrazine and 3-diphenylamine-9-carboxy-acenaphthopyrazine
[0051] (1) Synthesis of 3-diphenylamine-8-cyano-acenaphthopyrazine and 3-diphenylamine-9-cyano-acenaphthopyrazine
[0052]
[0053] The synthetic method is basically the same as (3) in Example 1, and what this reaction obtains is 3-diphenylamine-8-cyano-acenaphthopyrazine and 3-diphenylamine-9-cyano-acenaphthopyrazine The isomers of acene, the isomers cannot be separated by silica gel column, and the isomers can not be separated by NMR 1 HNMR showed that the ratio of isomers was about 1:1. NMR 1 HNMR (CDCl 3 ): δ7.10-7.16(m, 6H), 7.28-7.32(m, 4H), 7.45-7.48(m, 1H), 7.59(t, 1H), 7.83(d, 1H), 7.86-7.90(m , 1H), 8.25-8.28(m, 1H), 8.34-8.39(m, 2H), 8.54(s, 1H).FAB MS: m / e 446(M + ).
[0054] (2) Synthesis of 3-diphenylamine-8-carboxy-acenaphthopyrazine and 3-diphenylamine-9-carboxy-acenaphthopyrazine
[0055]
[0056] The synthetic method is b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com