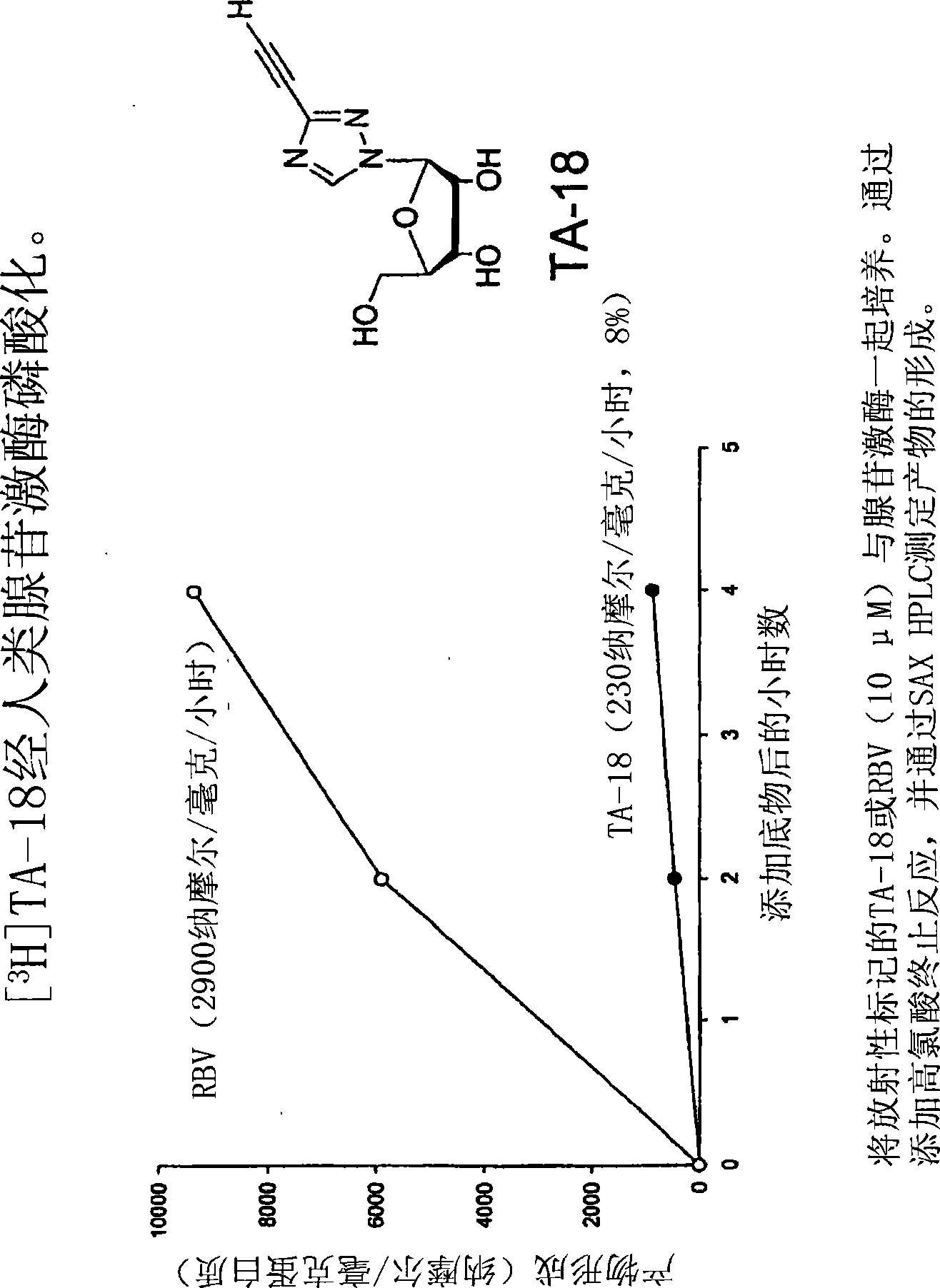
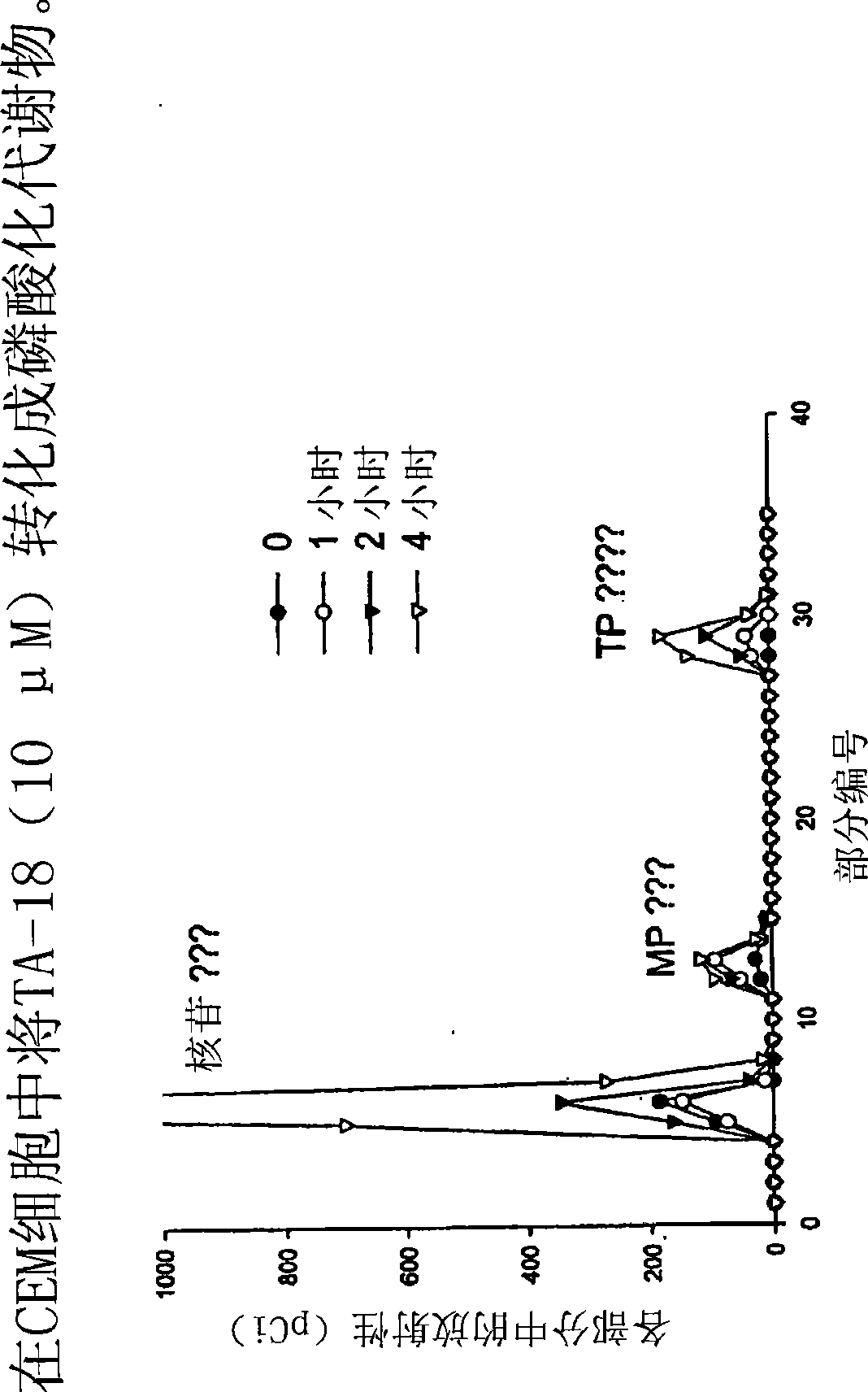
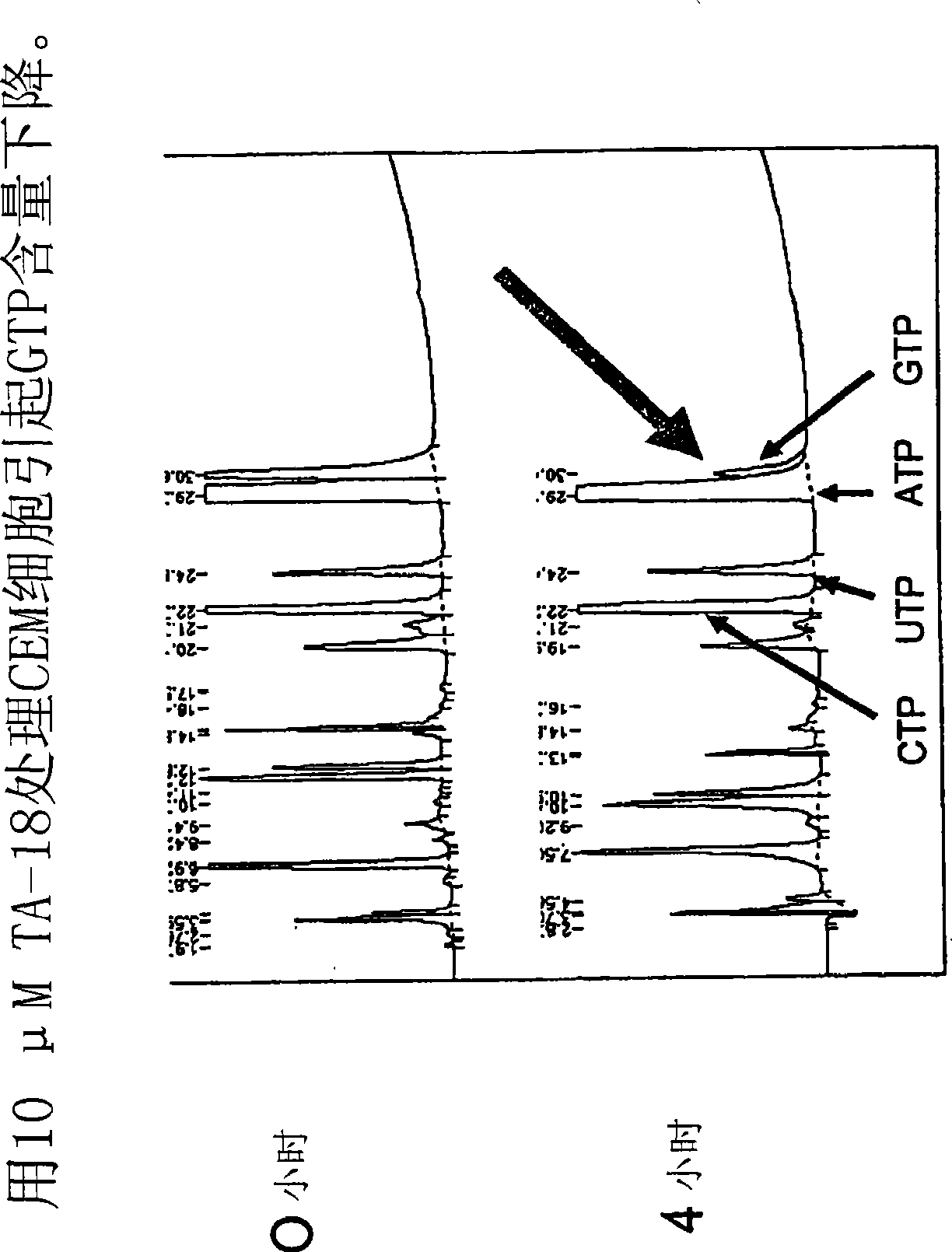
Azole nucleosides and use as inhibitors of rna and DNA varial polymerases
A technology of RNA viruses and compounds, applied in the field of azole nucleosides and as RNA and DNA virus polymerase inhibitors, can solve the problems of no better drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] The preparation of such prodrug derivatives is discussed in various literature sources (example: Alexander et al , Journal of Medicinal Chemistry (J.Med.Chem.) 1988, 31, 318; Aligas-Martin (Aligas-Martin) et al , PCTWO pp / 41531, p. 30). The nitrogen functionality converted during the preparation of these derivatives is the nitrogen atom(s) of the compounds of the invention.
[0066] Prodrug forms of the carboxyl-containing compounds of the present invention include esters (-CO 2 R), wherein the R group corresponds to any alcohol which is released in vivo at pharmaceutically acceptable levels by enzymatic or hydrolytic processes. Another prodrug obtained from the carboxylic acid form of the present invention may be Bodor et al , the quaternary salt structure described in Journal of Medicinal Chemistry (J.Med.Chem.) 1980,23,469:
[0067]
[0068] Pharmaceutically acceptable salts of the compounds of this invention include those derived from pharmaceutically acce...
example 1
[0177]
[0178] 2', 3', 5'-tris-(O-tert-butyldimethylsilyl)-inosine (TBS-I): at room temperature, inosine (5.36 g, 20 mmol) was dissolved in anhydrous DMF (100 mL) ) in TBS-Cl (18.1 g, 120 mmol) and imidazole (10.9 g, 160 mmol) for 48 hours. After concentration in vacuo, the mixture was washed with CH 2 Cl 2 Diluted to 200 and with water (4 washes), saturated NH 4 100 mL each of Cl (3 washes) and saturated NaCl followed by recrystallization in EtOAc gave a white crystalline solid (10.9 g, 17.8 mmol, 90%). FTIR (PTFE sheet, cm -1 )1706; 1 H NMR (400MHz, CDCl 3 -d) δ 13.0 (1H, s), 8.31 (1H, s), 8.21 (1H, s), 5.98 (1H, d, J=4.8Hz), 4.46 (1H, m), 4.26 (1H, m) , 4.09 (1H, m), 3.96 (1H, m), 3.75 (1H, m), 0.92-0.77 (27H, multiple singlets), 0.11-0.20 (18H, multiple singlets); 13 C NMR (400MHz, CDCl 3 -d) delta 159.3, 148.8, 145.3, 138.8, 124.8, 88.2, 85.2, 76.4, 71.5, 62.2, TBS - not listed.
example 2
[0180]
[0181] N 1 -(3-Fluorophenyl)-2',3',5'-tris-(O-tert-butyldimethylsilyl)-inosine (TBS-IA-3): oven dried Schlenk TBS-I (2.4 g, 4.0 mmol), 3-fluorophenylboronic acid (1.1 g, 8.0 mmol), and anhydrous Cu(OAc) were added to a Schlenk tube 2 (800.0 mg, 4.4 mmol), pyridine-N-oxide (800 mg, 4.0 mmol), triturated to 4 of molecular sieves (about 1 g) and a stir bar. The tube was then sealed with a rubber septum and evacuated and flushed with oxygen. Then add anhydrous pyridine (647 μL, 8.0 mmol) and molecular sieve dry CH 2 Cl 2 (20 mL) and the reaction was vigorously stirred at room temperature for 24 hours. followed by saturated NH in MeOH 4 The reaction was quenched with OH (0.5 mL in 5 mL individually), then diluted to 500 mL with hexane. The organics were washed with 250 mL portions of each of the following: water, saturated NH 4 Cl, 1M NaCl and saturated NaCl. Use Na 2 SO 4 Dry and concentrate in vacuo. by using CH 2 Cl 2 All compounds were purified by med...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com