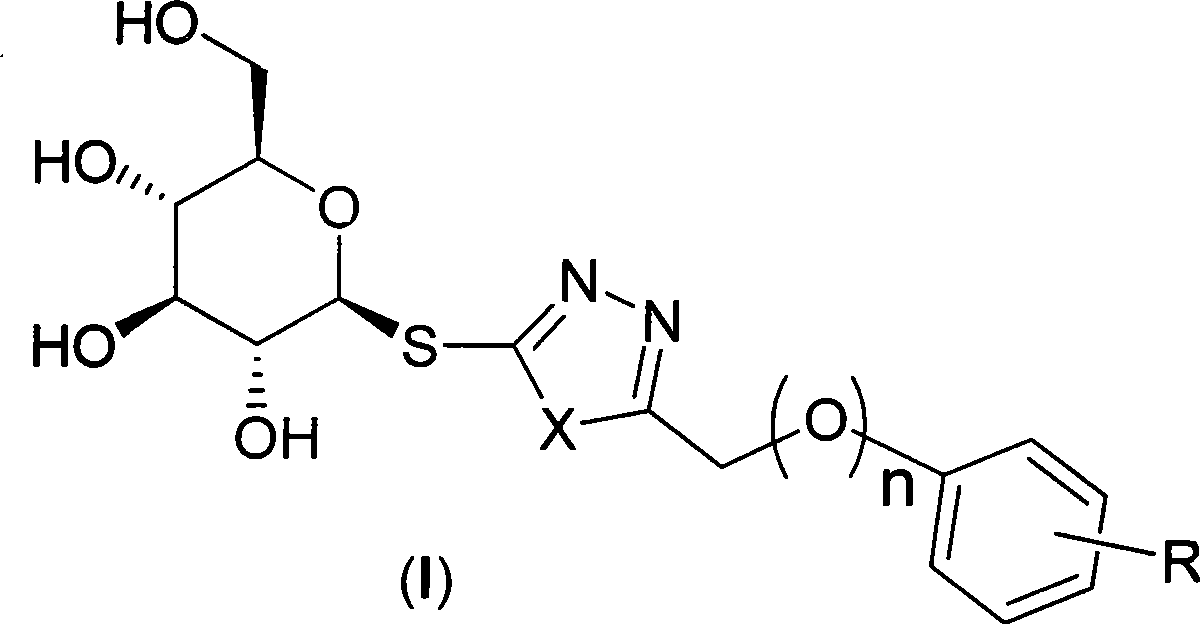
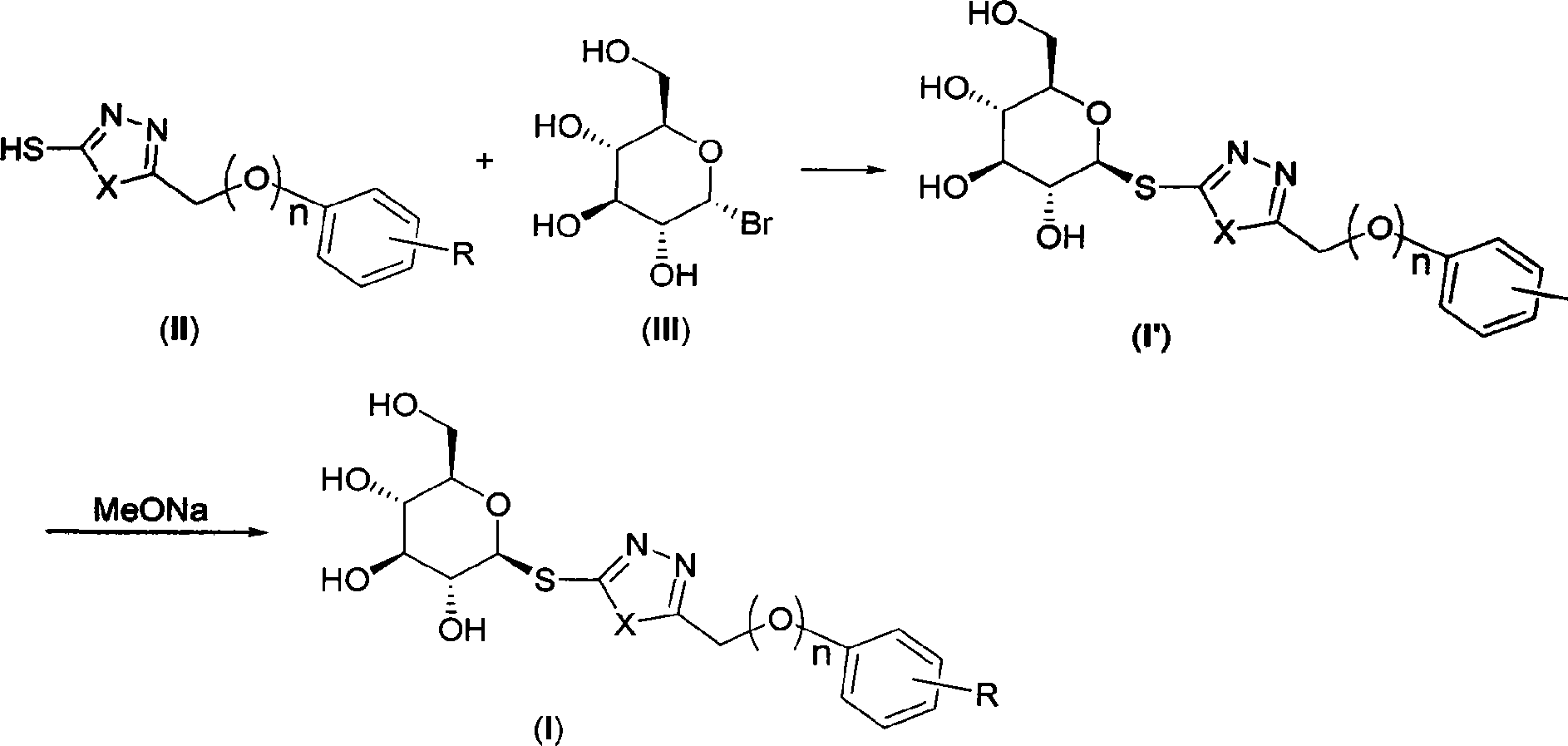
Sulpho-glucosan derivative and preparation method and application thereof
A glucopyranoside and thiol technology, which can be used in sugar derivatives, metabolic diseases, drug combinations, etc., and can solve problems such as hepatotoxicity and body weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Phenoxyacetylhydrazide (V-1)
[0039]
[0040] 18.0 g (0.1 mol) of compound IV-1 and 250 mL of absolute ethanol were added to a 500 mL round bottom flask, and 30 g (0.51 mol) of 85% hydrazine hydrate was added with stirring at room temperature. The reaction mixture was then warmed to reflux for 1 hour. After cooling to room temperature, the reaction system was concentrated to half of its original volume on a rotary evaporator, then stirred at room temperature for 1 hour, the crystals were collected by suction filtration, and compound V-1 was obtained after drying. Colorless crystals, 14.8g, yield 89%.
[0041] Compounds IV-1 and V-1 are one of the compounds having general formula IV and one having general formula IV, respectively.
Embodiment 2-10
[0043] With the operation of Example 1, replace Compound IV-1 in Example 1 with Compound IV-2 to IV-10 in the following table, and all the other operations are the same as Example 1 to obtain Compounds V-2 to V listed in the following table -5.
[0044] Compounds IV-2 to IV-10 and compounds V-2 to V-10 belong to two classes of compounds having general formula IV and having general formula V, respectively.
[0045] Example
Embodiment 11
[0047] 2-Mercapto-5-(phenoxymethyl)-1,3,4-thiadiazole (II-a-1)
[0048]
[0049] Add 4.98g (30mmol) compound V-1 and 100mL absolute ethanol to a 250mL round bottom flask, add 2.24g (40mmol) solid KOH under stirring at room temperature, stir at room temperature for 10 minutes, then dropwise add 3.80g (50mmol) to dissolve in 10 mL of absolute ethanol for dry CS 2After completion, the reaction system was stirred overnight at room temperature. A yellow solid was obtained by suction filtration, and dried to obtain compound VI-1 as a yellow solid. The obtained compound VI-1 was dissolved in 20 mL of concentrated sulfuric acid at room temperature, followed by stirring overnight at room temperature. The reaction system was poured into 200 mL of ice water, stirred for 10 minutes, suction filtered, and the filter cake was washed with water and dried to obtain 5.44 g of compound II-a-1, with a yield of 81%. IR(KBr), 3030, 1498, 1588, 1200cm -1 .
[0050] Compound VI-1 and compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com