Spirofluorene xanthene material, and preparation and use thereof
A spirofluorene xanthene and xanthene technology, which is applied in the field of spirofluorene xanthene materials and their preparation, and achieves the effects of mild conditions, high glass transition temperature and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
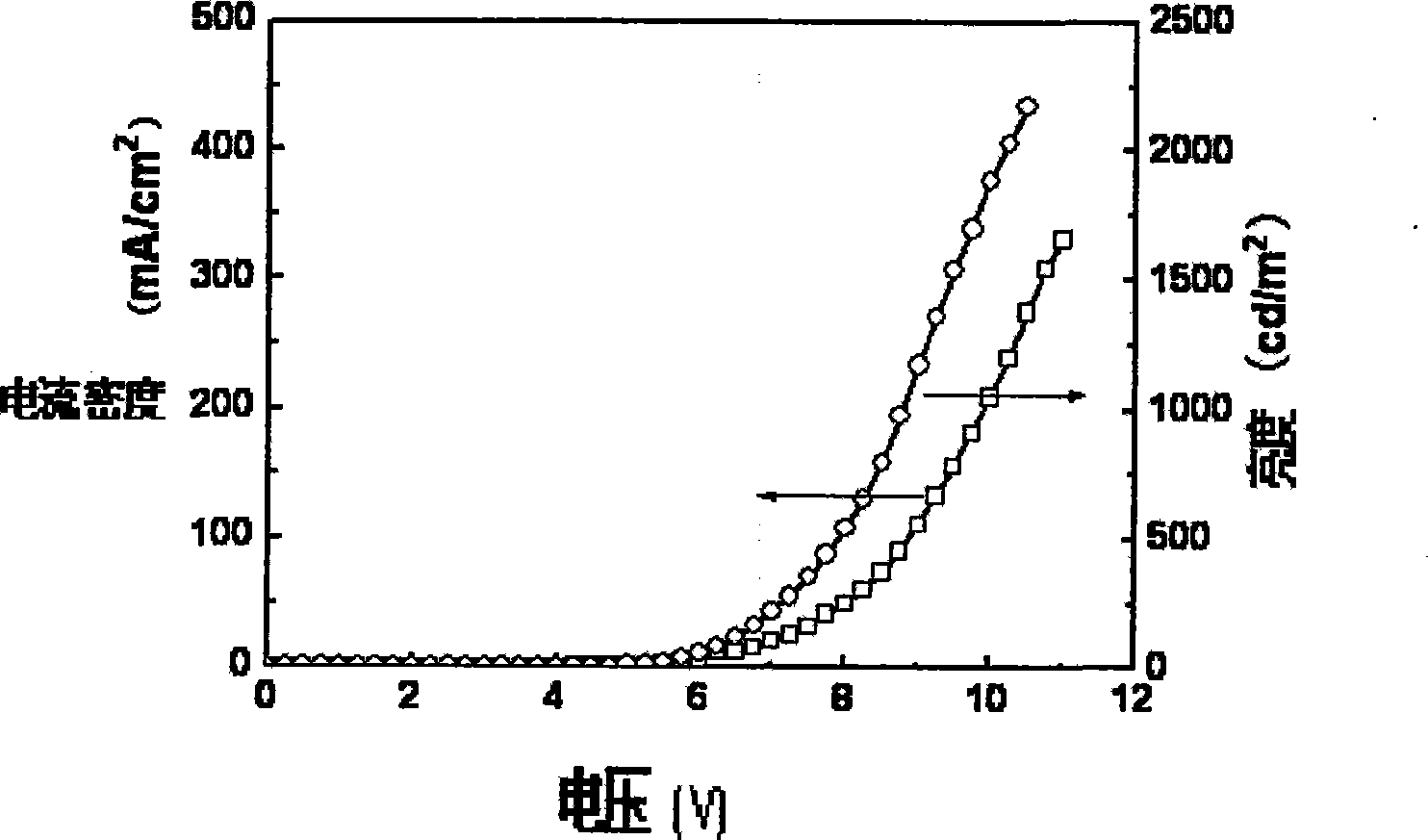
Examples
Embodiment 1、 2
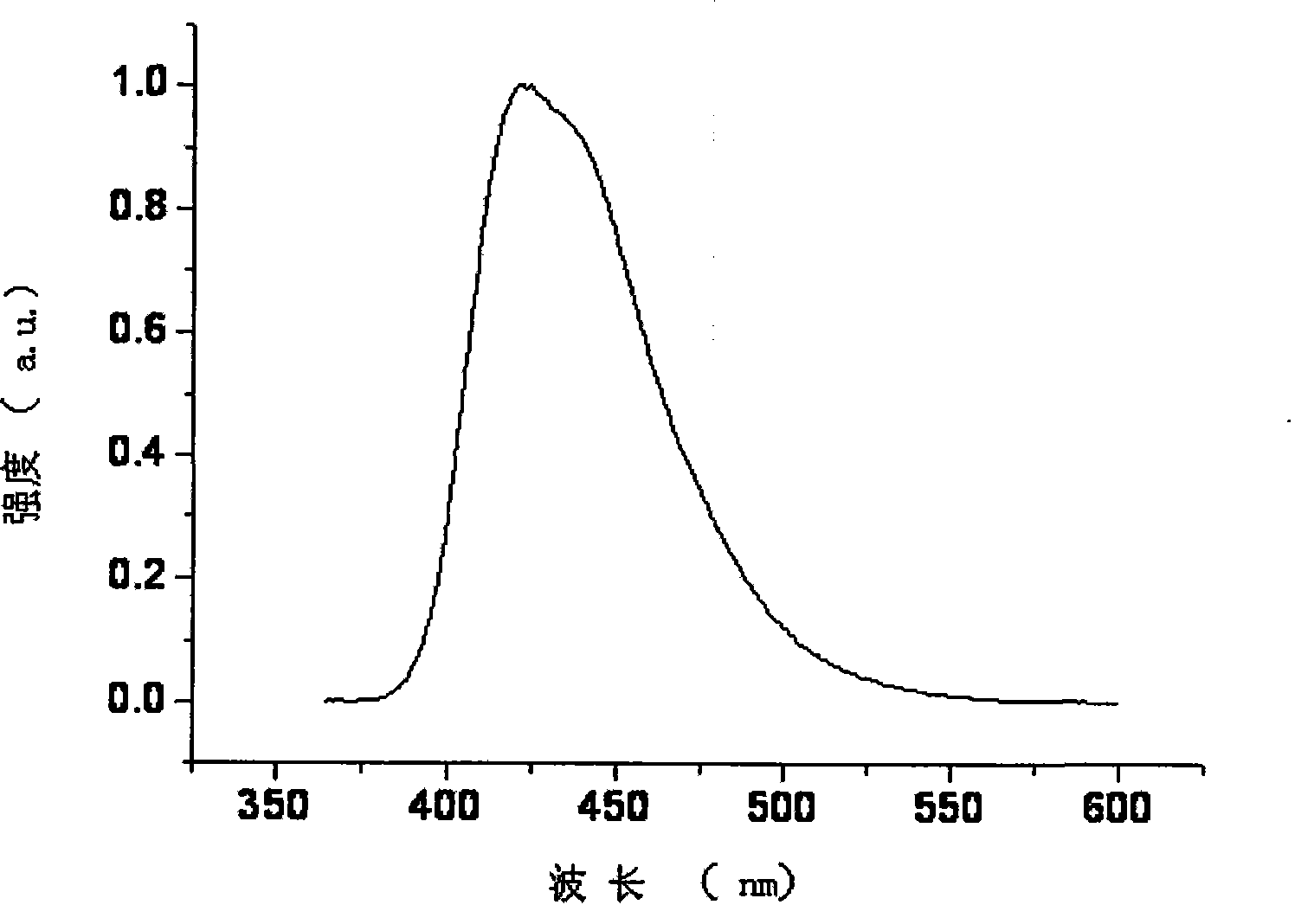
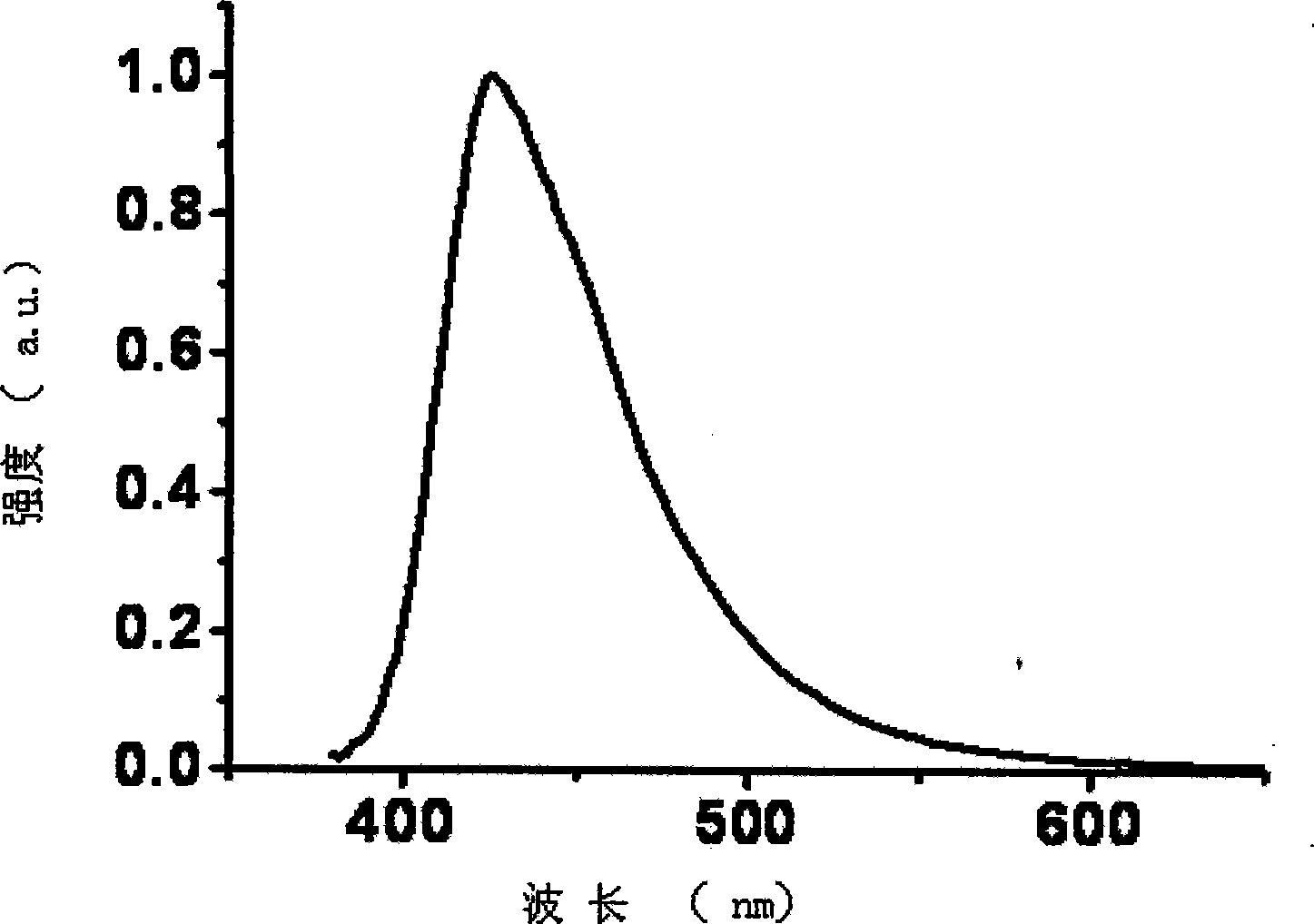
[0033] Embodiment 1, bispyrene spirofluorene xanthene material preparation:
[0034] 2,7-Dibromospiro[fluorene-9,9′-oxanthene]
[0035]
[0036] Take a 100 ml single-necked bottle, add 2,7-dibromofluorenone (6.523g, 19.3mmol, 1 equiv) and phenol (18g, 191.4mmol, 10 equiv), and add methanesulfonic acid (5.0mL, 7.41g, 77.2mmol, d=1.48g / ml, 4 equiv) were added, heated and stirred under anhydrous and oxygen-free conditions, and reacted at 140°C for 24 hours. After the reaction, cool the reaction, add water and dichloromethane under stirring, extract, and rotary evaporate under reduced pressure to obtain the crude product, which is recrystallized and purified with ethyl acetate, and dried to obtain 7.346 g of the product, with a yield of 78%. The purity is greater than 99, and the molecular weight measured by GCMS is 488 (M + ).
[0037] 2,7-Dipyrenespiro[fluorene-9,9′-oxanthene]
[0038]
[0039] Take 1-pyreneboronic acid (0.3673g, 1.35mmol) and 2,7-dibromospiro[fluorene...
Embodiment 2
[0040] Example 2, preparation of bis(dimethylfluorenyl) spirofluorene xanthene material:
[0041] 2,7-Dibromospiro[fluorene 9,9'-oxanthene] (see Example 1)
[0042] 2,7-Di-{2,2'-[9,9'-dimethylfluorene]}spiro[fluorene-9,9'-oxanthene]
[0043]
[0044]Take 2-[9,9'-dimethylfluorene]fluoreneboronic acid (0.5800g, 2.20mmol) and 2,7-dibromospiro[fluorene-9,9'-oxanthene] (1.0800g, 1.10mmol) , mixed and dissolved in the mixed solvent of 30ml toluene and tetrahydrofuran, add catalyst Pd(PPh3) 4, (255.2mg, 5mol%) and K 2 CO 3 (4.41ml, 2mol / L), the reaction system was refluxed under nitrogen protection for 48 hours, after the reaction was completed, extracted with dichloromethane, combined organic phase, anhydrous MgSO 4 After drying, the solvent was removed by rotary evaporation to obtain a crude product, which was obtained by column chromatography using a mixed solvent of petroleum ether and ethyl acetate as an eluent, with a yield of 86%.
Embodiment 3
[0045] Embodiment 3, preparation of bis(diphenylamino)spirofluorene xanthene material:
[0046] 2,7-Dibromospiro[fluorene-9,9'-oxanthene] (see Example 1)
[0047] 2,7-bis[N,N-diphenyl]spiro[fluorene-9,9′-oxanthene]
[0048]
[0049] Take diphenylamine (0.3462g, 2.0485mmol), 2,7-dibromospiro[fluorene-9,9'-oxanthene] (0.5023g, 1.023mmol), potassium carbonate (0.1403g, 1.024mmol), iodide Cuprous (0.0411g, 0.2158mol), small pieces of 18-crown-6 and dichlorobenzene (16ml), the reaction system was refluxed for 24 hours under the protection of nitrogen. Then, the dichlorobenzene in the system was distilled off under reduced pressure, and the remaining product was extracted with dichloromethane, the organic phases were combined, dried with anhydrous MgSO4, and the solvent was removed by rotary evaporation to obtain the crude product, using a mixed solvent of petroleum ether and ethyl acetate Column chromatography as eluent gave white product in 73% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com