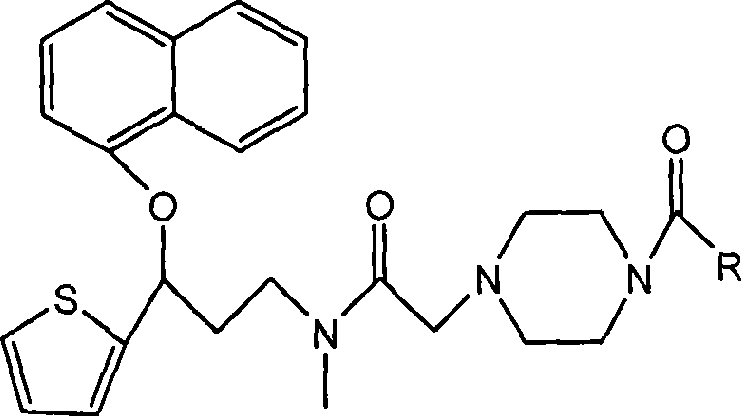
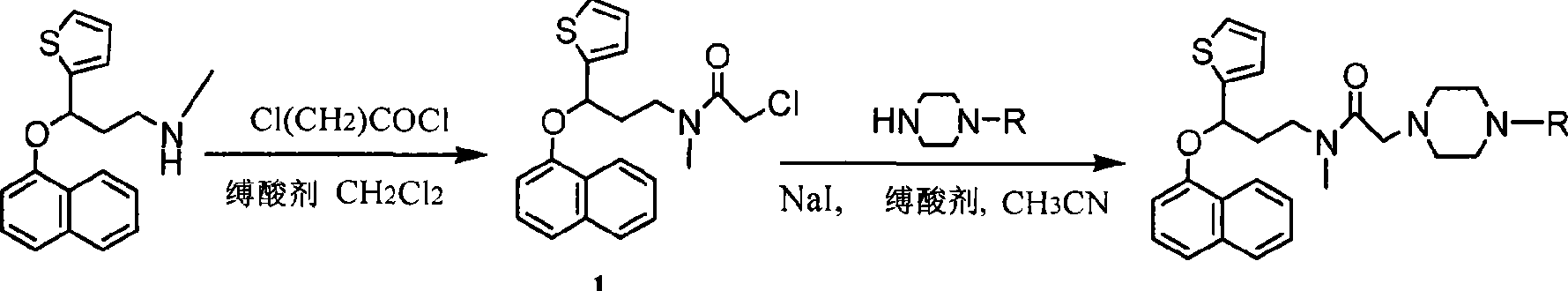
Duloxetine derivative and preparation thereof
A technique for duloxetine and its derivatives, which is applied in the field of duloxetine derivatives and its preparation, can solve the problems of long drug lag period, poor tolerance and safety, poor curative effect, etc., and achieves simple and fast preparation process easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: 4-methylbenzoacryloyl chloride
[0022] Add p-methylcinnamic acid (9.732g, 0.06mol) and 2 drops of N,N-dimethylformamide (DMF) into the three-necked flask, add 100ml of toluene as a solvent, slowly drop (14.2752g, 0.12mol) chlorinated For sulfoxide, the temperature was slowly raised to 100°C and refluxed for 3 hours, vacuum rotary evaporation (vacuum degree 10mmHg, the same as the following examples) to remove toluene and excess thionyl chloride, oil pump vacuum distillation to collect 135-136°C / 5mmHg components 10.306 g of white solid was obtained with a yield of 95.0%. mp: 60-62°C.
[0023] IR(KBr)v(cm -1 ): 3085, 2923, 1741, 1601, 1510, 806, 771.
Embodiment 2
[0024] Example 2: 4-methoxyphenylacryloyl chloride
[0025] Add p-methoxycinnamic acid (21.381g, 0.12mol) to the three-necked flask, catalytic amount of N,N-dimethylformamide (DMF), solvent toluene 160ml, slowly dropwise add thionyl chloride (28.554g, 0.24 mol), the temperature was slowly raised to 100°C and refluxed for 3 hours, toluene and excess thionyl chloride were removed by vacuum rotary evaporation, and the 144-145°C / 2mmHg fraction was collected by oil pump vacuum distillation to obtain 22.286 g of bright yellow solid, with a yield of 93.942 %. mp: 40-44°C.
[0026] IR(KBr)v(cm -1 ): 3052, 2844, 1688, 1600, 1514, 862, 775.
Embodiment 3
[0027] Example 3: 4-chlorophenylacryloyl chloride
[0028] Add p-chlorocinnamic acid (18.204g, 0.10mol) in the three-necked flask, catalytic amount of N,N-dimethylformamide (DMF), solvent toluene 100ml, slowly add thionyl chloride (23.793g, 0.2mol) dropwise , the temperature was slowly raised to 100°C and refluxed for 3 hours, the toluene and excess thionyl chloride were removed by vacuum rotary evaporation, and the 118-120°C / 3mmHg fraction was collected by oil pump vacuum distillation to obtain 19.301g of white solid with a yield of 96.55%. mp: 61-63°C. IR(KBr)v(cm -1 ): 3034, 2970, 1699, 1592, 1490, 846, 736.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com