Monoclonal antibody, gene encoding the antibody, hybridoma, pharmaceutical composition, and diagnostic reagent
A monoclonal antibody and composition technology, which can be used in drug combinations, anti-tumor drugs, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., and can solve the problem of not developing antibodies.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0074] Hereinafter, the present invention will be described in more detail with reference to Examples. However, the present invention is not limited to these examples without departing from the scope of the present invention.
[0075] (1) Preparation of hybridomas by cell fusion of lymphocytes obtained from local lymph node cancer of cancer patients and mouse myeloma
[0076] (1)-1: Preparation of lymphocytes
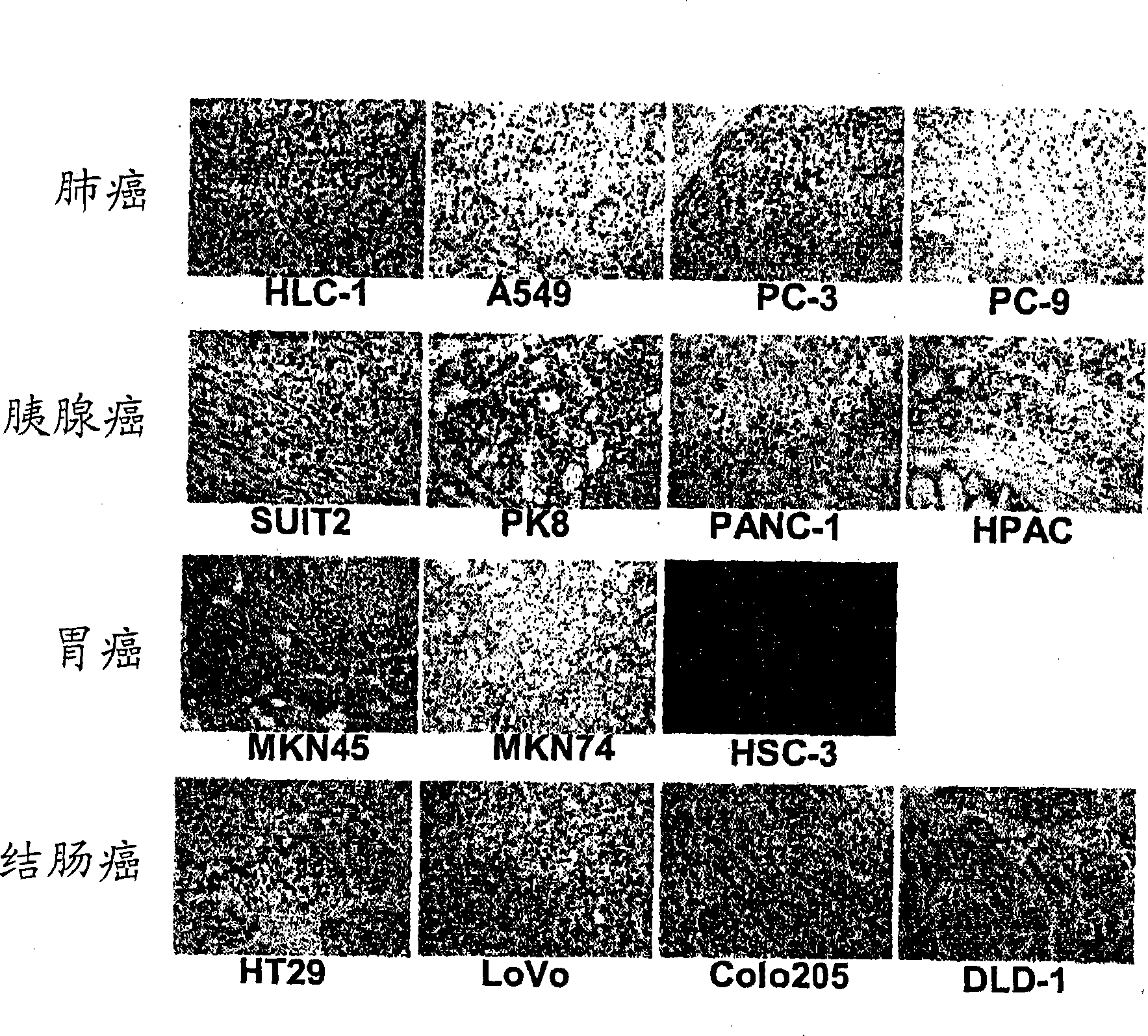
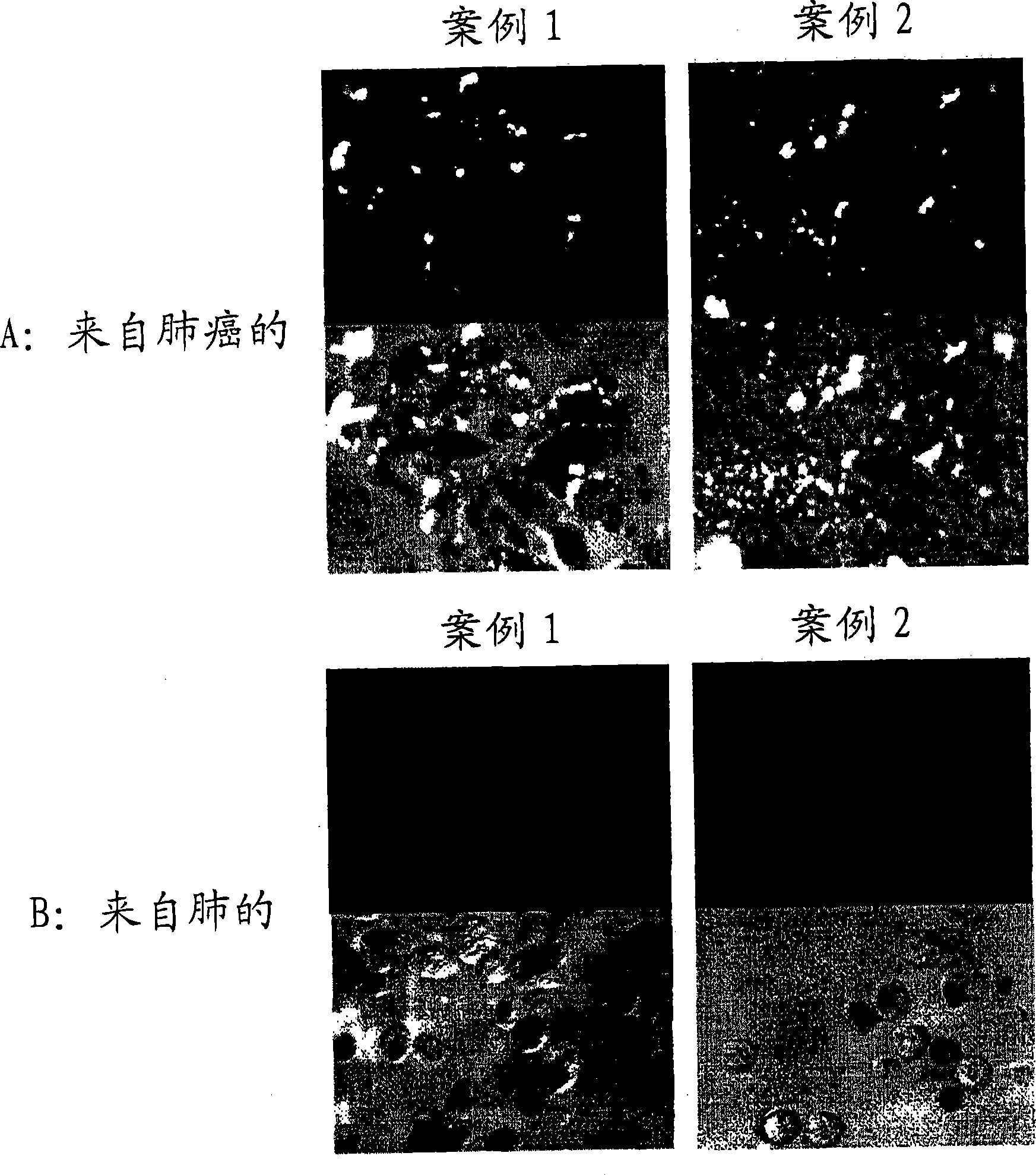
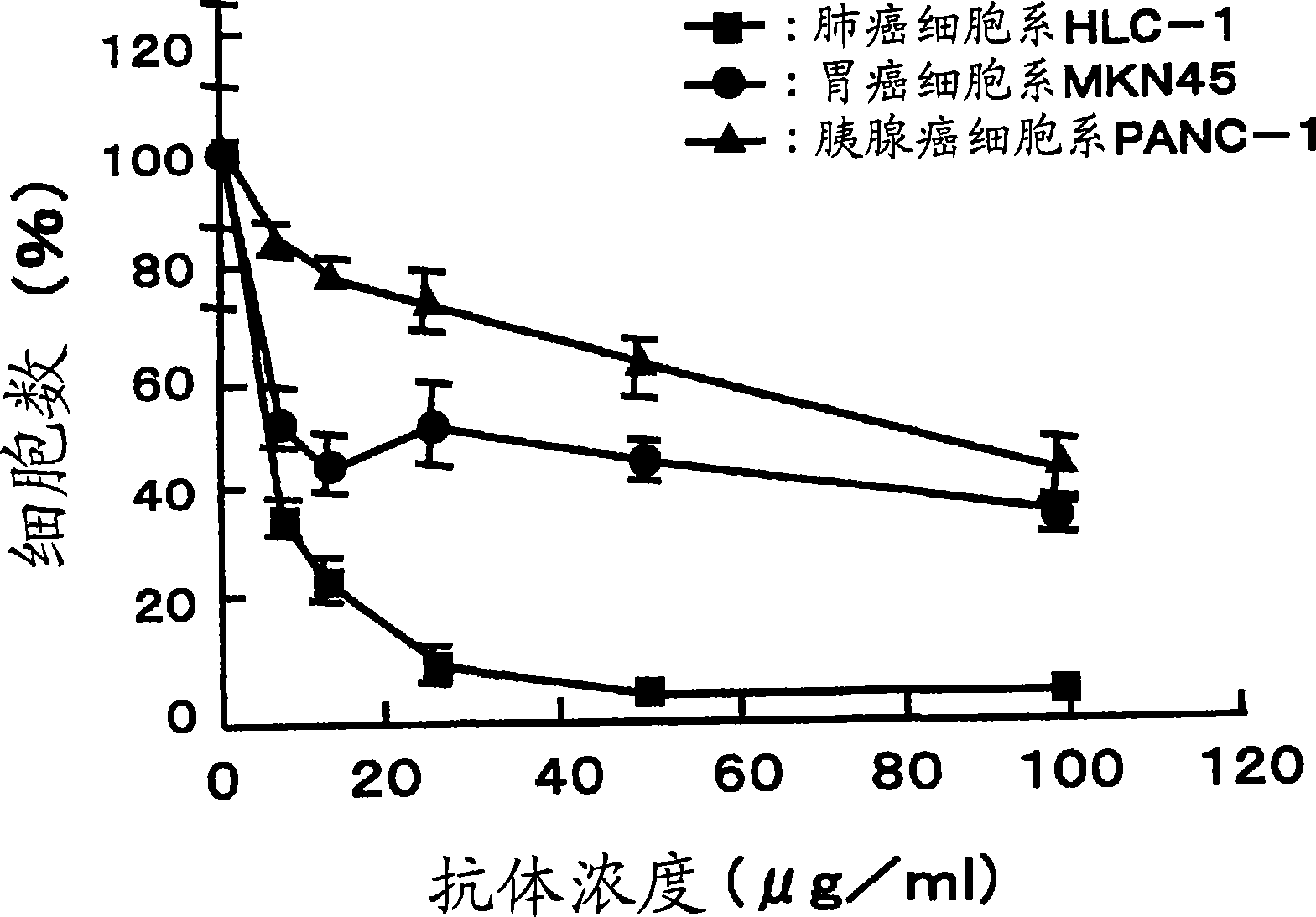
[0077] Carcinoma of regional lymph nodes resected from patients with gastric The isolated lymphocytes were dispersed on the metal mesh. Centrifuge the cell suspension at 3,000 rpm for 5 min to obtain approximately 4.8 × 10 7 A lymphocyte obtained from a carcinoma of a regional lymph node.
[0078] (1)-2: cell fusion
[0079] According to the standard method (Cancer Research (Cancer Research), Vol. 45, p. 263, 1985), the lymphocytes obtained from regional lymph node carcinoma and mouse myeloma cells (the number thereof) were separated with polyethylene glycol 1500 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com