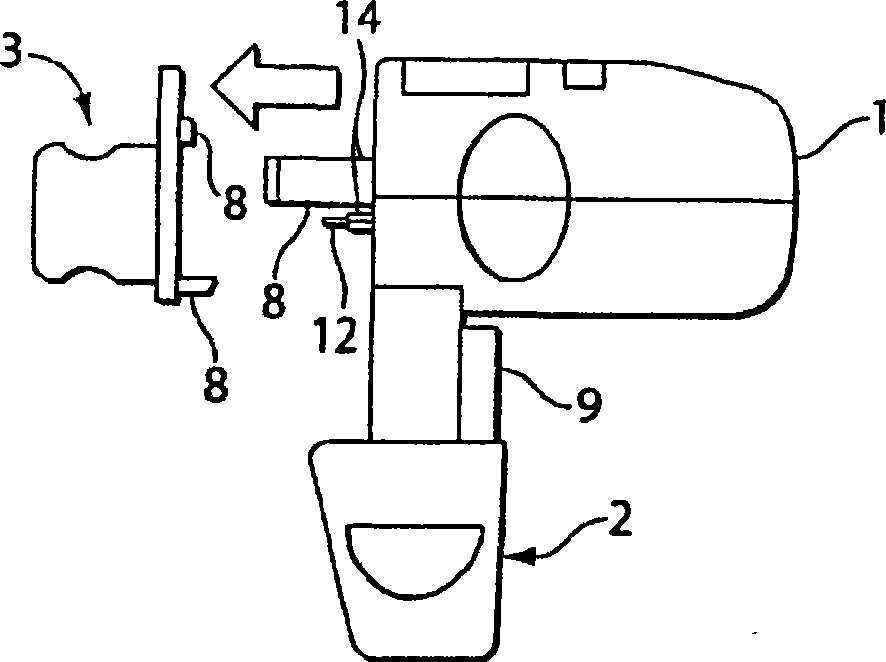
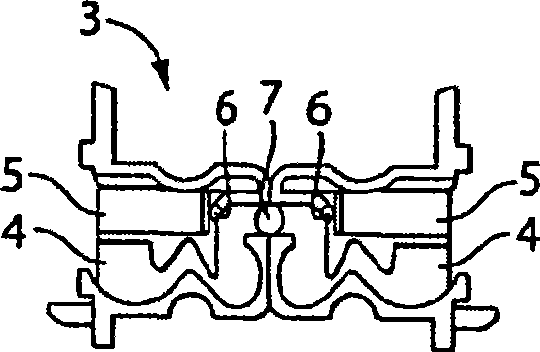
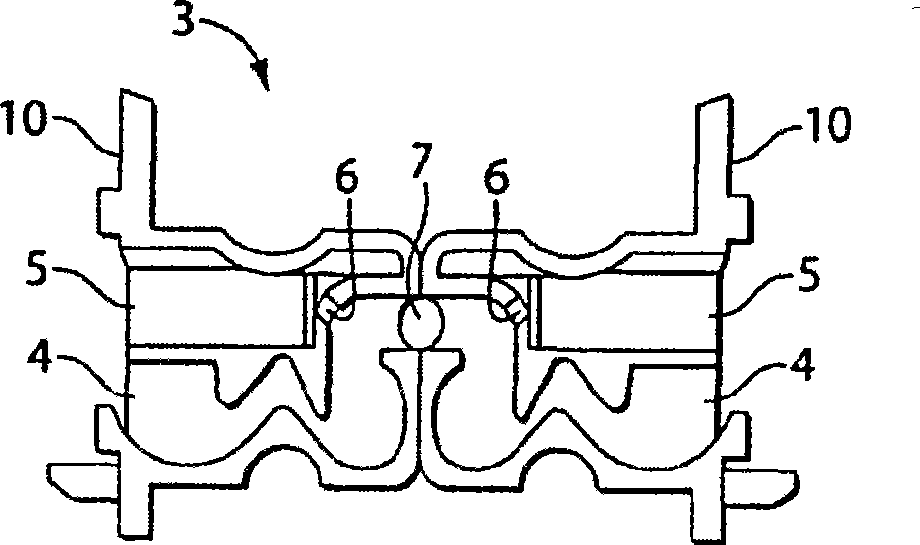
Dry powder inhaler device
An inhaler and equipment technology, applied in the field of improvement of dry powder inhaler equipment, can solve the problems of difficulty in achieving the force, inability to inhale, resistance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0089] In this example, the fine particle fraction (average aerodynamic diameter of 4.4 microns or less) as a percentage of the dose delivered from a dry powder inhaler according to the invention is compared with a device with a mouthpiece, which The tubing has a single passage including the agglomerate separation device described in US Patent 6,182,655.
[0090] Measurements were performed by Dose Unit Sampling Apparatus (DUSA). This device is well known in the art and is described in USP Chapter 24 .
[0091] The device was operated at different airflow rates, producing in each case a pressure drop of 4 kPa over the specific dry powder inhaler tested.
[0092] The comparative device according to U.S. Patent 6,182,655 with a single access through the mouthpiece gave a delivered dose of 8.8 mg, and a fine particle fraction of 36.8% (RSD of FPF of the delivered dose = 0.5%; n = 3) points (less than or equal to 3.3 microns).
[0093] The four devices were identical to the com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com