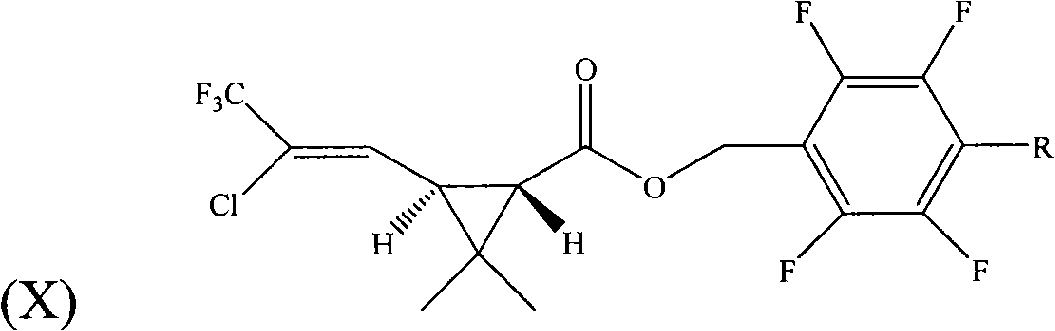
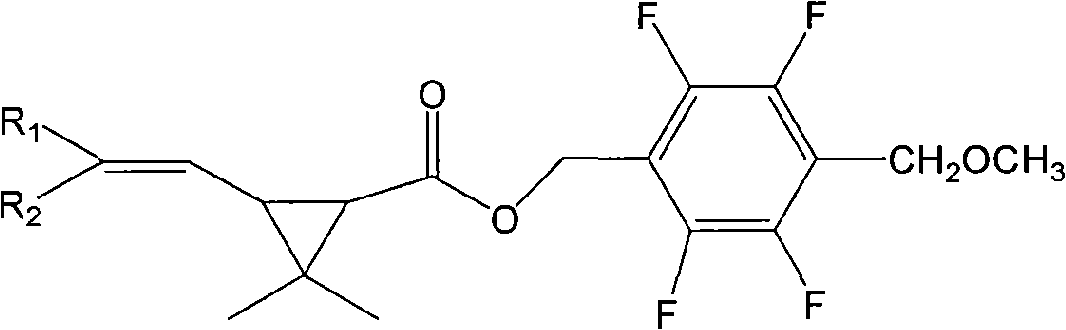
Pyrethroid compounds, preparation and use thereof
A technology of pyrethroids and compounds, which is applied in the field of pyrethroid compounds, can solve problems such as no compound research reports, and achieve the effect of quickly killing pests
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
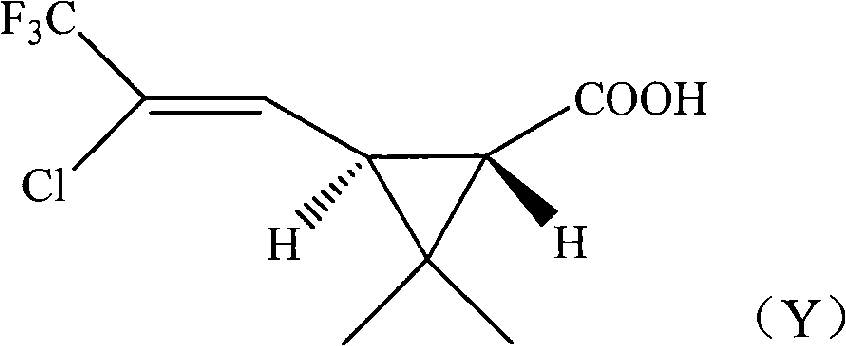
[0043] Preparation Example 1: Resolution of trans-2,2-dimethyl-3-(2-chloro-2-trifluoromethylvinyl)cyclopropanecarboxylic acid
[0044] In a 1000ml four-necked bottle, drop into trans 2,2-dimethyl-3-(2-chloro-2-trifluoromethylvinyl)cyclopropanecarboxylic acid 100.0g, d-chloramphenicol 100.0 g, dissolved in 500ml of toluene, stirred after throwing in, heated to 110°C for reflux reaction for 1 hour, then cooled to 40°C within 3 hours, kept for 1 hour, then cooled to 10°C within 2 hours, kept for 0.5 hours, at this time A large number of crystals were precipitated. Filter, add 100g of 10% hydrochloric acid to the mother liquor obtained to acidify to pH 2-3, separate layers, wash the oil layer to near neutrality, heat to 100°C under 10mmHg negative pressure to remove the solvent toluene, and obtain the dextrorotatory trans ( 1R,3S)-2,2-Dimethyl 3-(2-chloro-2-trifluoromethylvinyl)cyclopropanecarboxylic acid 45.5g, dextrorotatory active body ee value 95%.
preparation Embodiment 2
[0045] Preparation Example 2: Resolution of trans-2,2-dimethyl-3-(2-chloro-2-trifluoromethylvinyl)cyclopropanecarboxylic acid
[0046] In a 1000ml four-necked bottle, put 100.0g of trans-2,2-dimethyl-3-(2-chloro-2-trifluoromethylvinyl)cyclopropanecarboxylic acid, dextrorotatory PTE ((+ ) β-p-methylphenyl-α-phenylethylamine) 125.0g, dissolved in 400ml toluene, stirred after throwing in, heated to 110°C for reflux reaction for 1 hour, then cooled to 60°C within 3 hours, and kept for 1 hour, Cool to 20°C within 2 hours and keep warm for 1 hour. At this time, a large amount of crystals precipitated, and the crystals were obtained by filtration, acidified to pH 2 by adding 200g of 5% hydrochloric acid, and extracted by adding 400ml of toluene at the same time. Toluene is removed by heating to 100°C under negative pressure of 10mmHg to obtain dextrorotatory trans (1R,3S)-2,2-dimethyl 3-(2-chloro-2-trifluoromethylvinyl) ) Cyclopropanecarboxylic acid 45.2g, dextrorotatory effective b...
preparation Embodiment 3
[0047] Preparative Example 3: Acid Chlorination of (1R,3S)-2,2-Dimethyl 3-(2-Chloro-2-trifluoromethylvinyl)cyclopropanecarboxylic Acid
[0048] In a 1000ml four-necked bottle, drop (1R, 3S)-2,2-dimethyl 3-(2-chloro-2-trifluoromethylvinyl) cyclopropanecarboxylic acid obtained in Preparation Example 1 242.5g (1mol ee value 95%), dissolved in 600ml toluene, stirred after throwing in, warming up to 50°C, adding SOCl dropwise 2 142g (1.2mol), dropwise completed within 2 hours, then heated up to 60°C, and kept warm for reaction. After completion of the reaction, heat to 80°C under 30mmHg negative pressure to remove solvent toluene, then rectify under 10mmHg negative pressure, and collect 60°C-75°C fractions to obtain (1R,3S)-2,2-dimethyl-3-( 2-Chloro-2-trifluoromethylvinyl)cyclopropanecarboxylic acid chloride 249.6.1g, yield 94.3%, dextrorotatory active body ee value 95%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com