Process for synthesizing D-alpha-(6-methyl-4-hydroxyl nicotinamide base)p-hydroxyphenylacetic acid
A technology of hydroxynicotinamide and ethyl hydroxyphenylacetate is applied in the synthesis field of pharmaceutical intermediates, can solve the problems of high equipment and operation requirements, harsh reaction conditions, long synthesis routes, etc., and achieves easy control of process parameters. , the effect of strong quality and cost, high product yield
Active Publication Date: 2010-11-10
QILU ANTIBIOTICS PHARMA
View PDF1 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The structure of the compound is complex, the synthetic route is long, and the reaction conditions are harsh, thereby restricting the production of cefpiramide bulk drug
Patent JP54-030197 introduces a synthetic method of D-α-(6-methyl-4-hydroxynicotinamide) p-hydroxyphenylacetic acid (I) in the form of a reference example, but because its intermediates need to pass through Dry hydrogen chloride gas is made into hydrochloride, which requires high equipment and operation, which is not conducive to large-scale industrial production
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
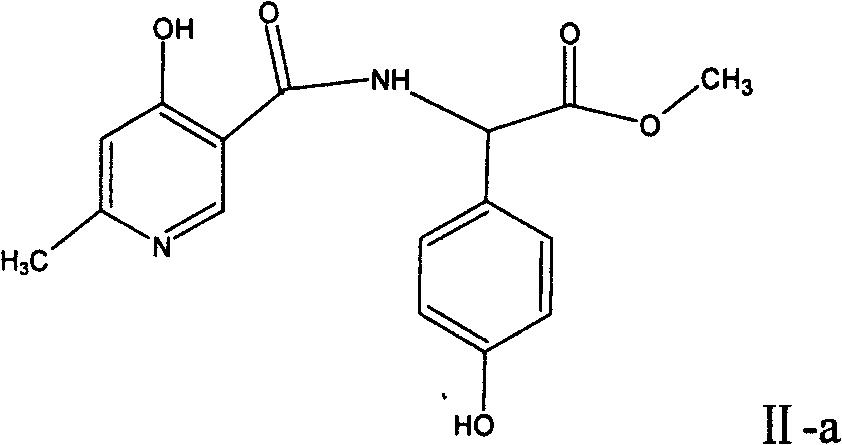
The invention relates to a method for synthesizing an intermediate of cephalosporins antibiotics cefpiramide, D-alpha-(6-methyl-4-hydroxy nicotinamido)-p-hydroxyphenyl acetic acid. The method comprises the steps of: reacting carboxyl group protected D-p-hydroxyphenyl glycine and anhydride or acyl chloride of 4-hydroxy-6-methylnicotinic acid to obtain acylated product, and hydrolyzing to remove the protecting group to obtain the target product D-alpha-(6-methyl-4-hydroxy nicotinamido)-p-hydroxyphenyl acetic acid. The method has the advantages of easy and feasible operation, and applicability to large scale production.
Description
Synthesis of D-α-(6-methyl-4-hydroxynicotinamide) p-hydroxyphenylacetic acid technical field The invention relates to a synthesis method of D-α-(6-methyl-4-hydroxynicotinamide) p-hydroxyphenylacetic acid, an acid intermediate at the 7-position of cefpiramide, a cephalosporin antibiotic, and belongs to the technical field of synthesis of pharmaceutical intermediates . Background technique The chemical name of cefpiramide (cefpiramide) is (6R, 7R)-7-[(R)-2-(4-hydroxy-6-methyl-3-pyridinecarbonylamino)-2-(p-hydroxyphenyl)acetyl Amino]-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-5-thia-1-azabicyclo[4,2,0] Oct-2-ene-2-carboxylic acid. Its structural formula is: Cefpiramide is a third-generation cephalosporin antibiotic jointly developed by Sumitomo Pharmaceutical Co., Ltd. and Yamanouchi Pharmaceutical Co., Ltd. It was first listed in Japan in 1985 and has been included in the American, Japanese, British and European Pharmacopoeias. It is characterized by broad anti...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D213/82
CPCY02P20/55
Inventor 赵振华董付敏王勇进
Owner QILU ANTIBIOTICS PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com