Activating agent of stem cells and/or progenitor cells
A stem cell and progenitor cell technology, applied in the direction of non-embryonic pluripotent stem cells, cell culture support/coating, animal cells, etc., can solve problems such as the evaluation of batroxobin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
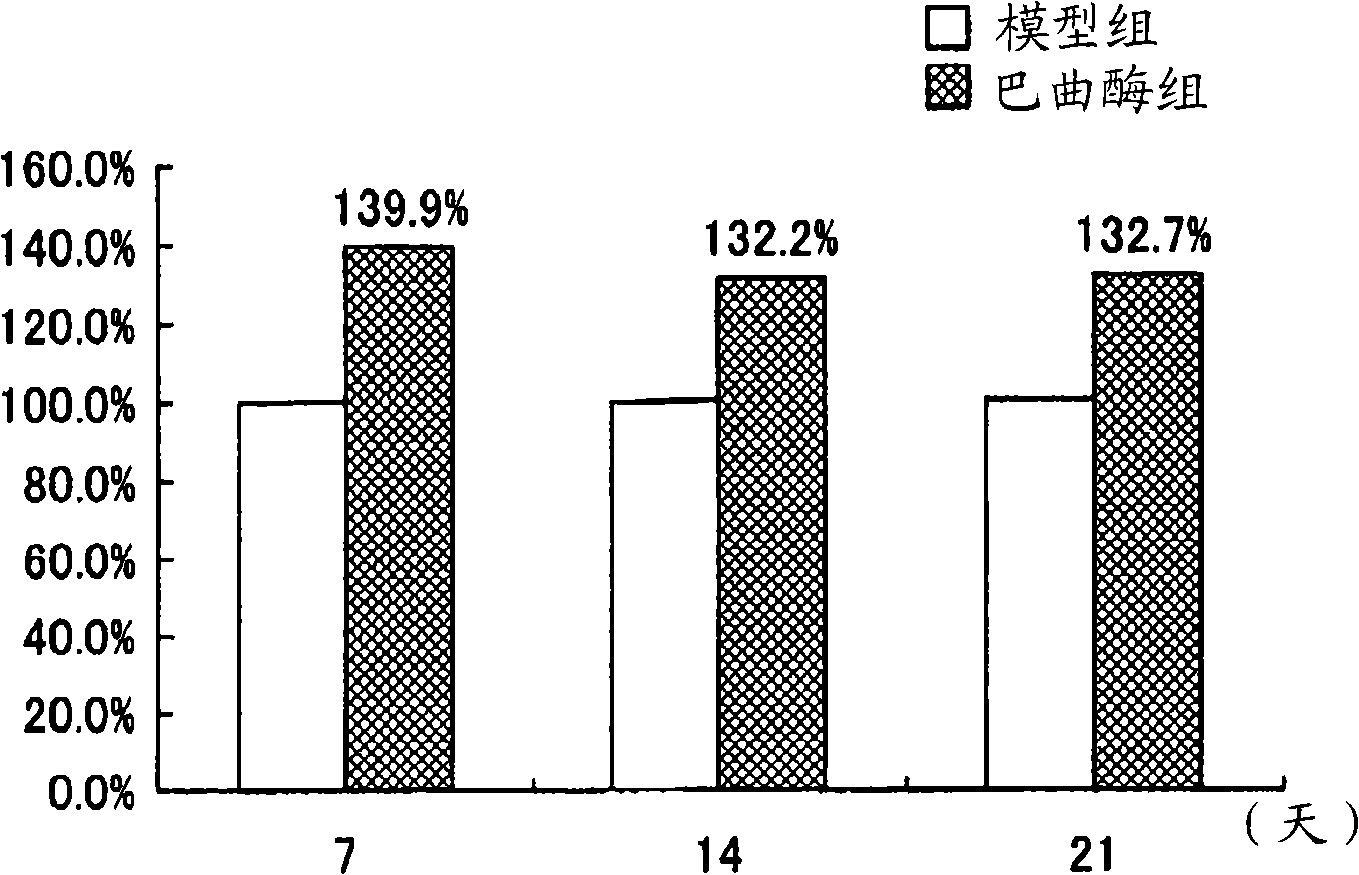
[0114] The activation effect of batroxobin on CD34 positive mononuclear cells derived from umbilical cord blood
[0115] In this example, the activation effect of batroxobin on CD34-positive mononuclear cells derived from umbilical cord blood was evaluated in vitro.
[0116] Furthermore, it is known that cells consistent with CD34-positive mononuclear cells present in umbilical cord blood consist of vascular EPCs and mesenchymal stem cells (Murohara T et al.: Transplanted cordblood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest 105: 1527 -1536, 2000). Therefore, it can be said that the human umbilical cord blood-derived CD34-positive mononuclear cells evaluated in this example are vascular EPCs and mesenchymal stem cells.
[0117] experimental method:
[0118] (1) Collection of human umbilical cord blood mononuclear cells
[0119] Normal pregnancy with full-term delivery was selected, and 40-60 ml of umbilical cord blood was coll...
Embodiment 2
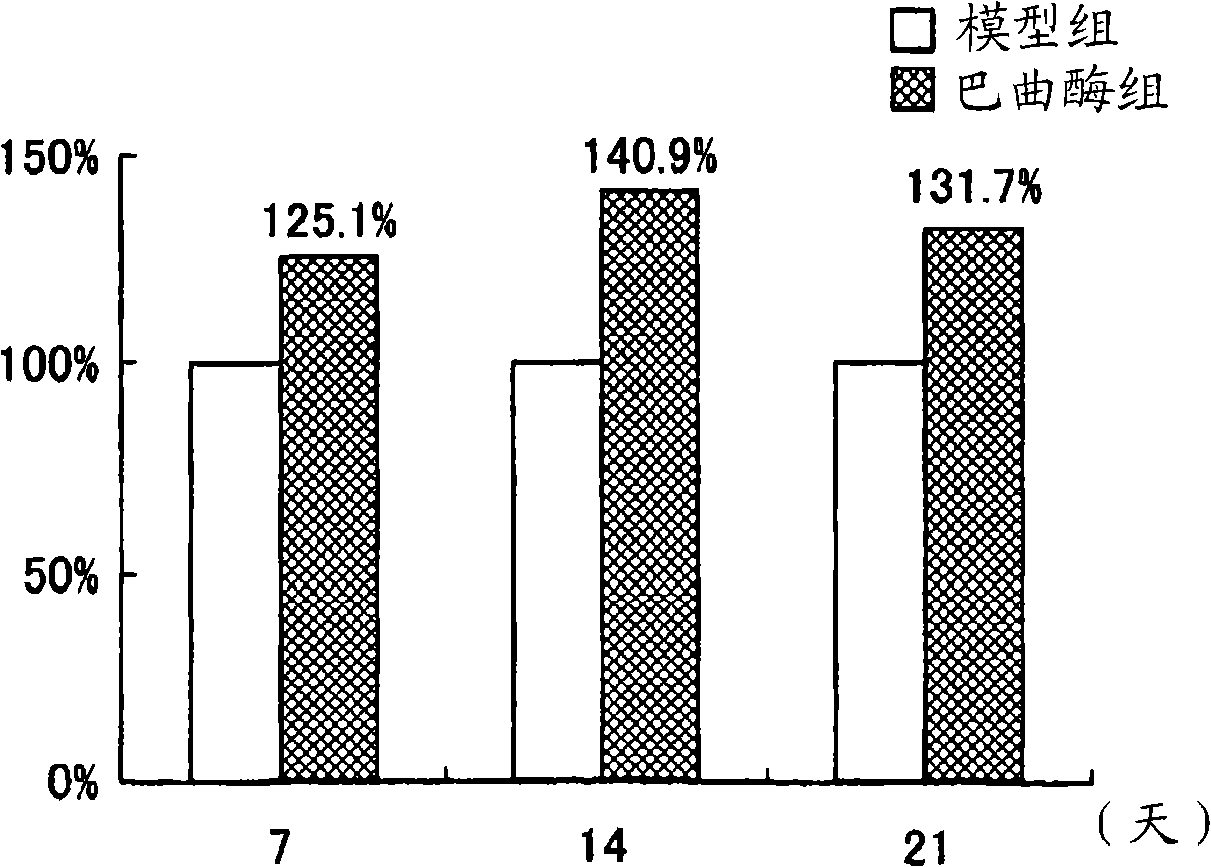
[0131] Activation of CD34-positive cells, CD34-positive / CD31-positive cells and vascular endothelial-cadherin-positive cells in peripheral blood of patients with deep venous thrombosis of lower extremities, and the function recovery effect of batroxobin on injured tissues in patients with deep venous thrombosis of lower extremities
[0132] In the present embodiment, batroxobin is used in patients suffering from a kind of vascular disease-lower extremity deep venous thrombosis (lower extremity DVT), to evaluate batroxobin on CD34 positive cells, CD34 positive / CD31 positive cells and The activating effect of VE-Cadherin positive cells, and the regenerative effect of batroxobin on thrombus-injured blood vessels.
[0133] In addition, those cells considered to be CD34-positive mononuclear cells present in peripheral blood are known to be vascular EPCs and mesenchymal stem cells (Zhao Y et al.: A human peripheral blood monocyte-derived subset acts as pleuripotent stem cells.Proc N...
Embodiment 3
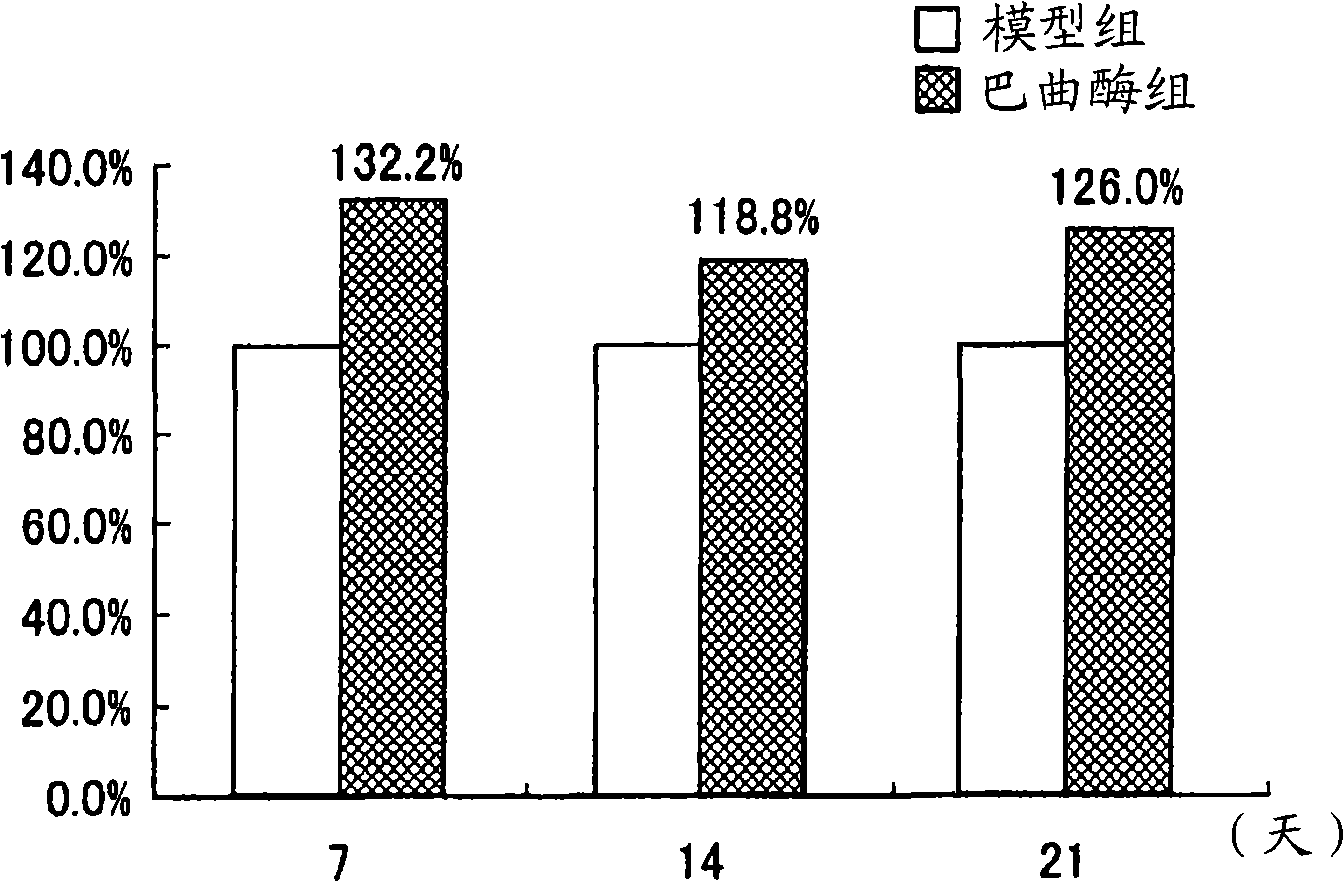
[0186] Effect of batroxobin on neural stem cells and / or neural progenitor cells in cerebral ischemia / reperfusion injury model
[0187] The role of life and neurological recovery
[0188] In this example, the effect of batroxobin on the activation of neural stem cells and / or neural progenitor cells and the recovery of neural function was evaluated using a rat cerebral ischemia / reperfusion injury model.
[0189] experimental method:
[0190] (1) animal
[0191] Male Sprague-Dawley rats (Shanghai Animal Center, Chinese Academy of Medical Sciences, Shanghai, China) aged 12 weeks and weighing 250-280 g were used for the experiment after acclimatization for 1 week.
[0192] (2) Establishment of cerebral ischemia / reperfusion injury model
[0193] First, a middle cerebral artery occlusion model was established according to the method of Longa EZ et al. (Longa EZ et al.: Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84-91, 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com