Oxygen-carrying blood substitute preparations
A blood substitute and oxygen-carrying technology, which is applied in the direction of blood diseases, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of increasing production costs and achieve the effect of avoiding cost increases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1. Stability of liposomal dispersions containing L-Cys in glass vials
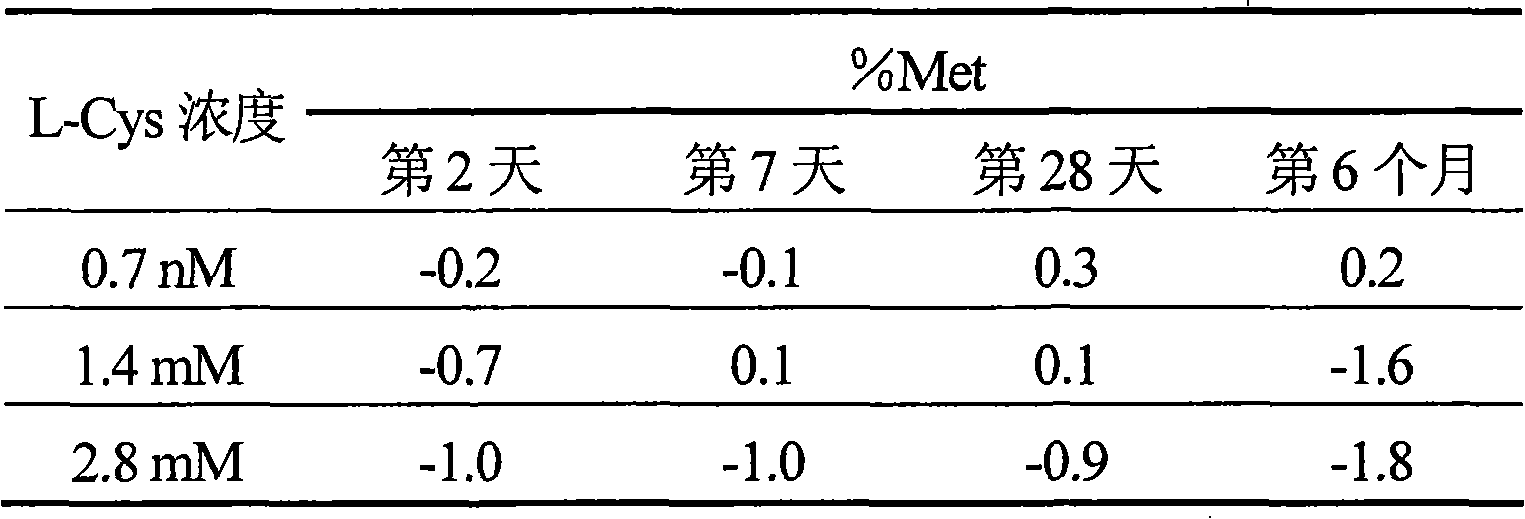
[0073] 10 mL glass vials (24 mm cylinder diameter, 40 mm height and 12.5 mm aperture) were filled with the sample solutions prepared in step 2 containing 0.7 nM, 1.4 mM or 2.8 mM L-Cys and were capped with rubber stoppers and aluminum caps seal. The filling process is carried out in an atmosphere with an oxygen concentration of less than 0.1%. Each vial was stored at 25°C for 6 months. The stability of hemoglobin encapsulated in liposomes was determined at days 2, 7, 28 and 6 months as percent methemoglobin (% Met) using the method described above. The results are shown in Table 1 below.
[0074] Table 1. % Met of liposome-coated hemoglobin in glass vials
[0075]
[0076] As shown in Table 1, %Met remained essentially unchanged after 6 months of storage at 25°C.
Embodiment 2
[0077] Example 2. Under hypoxia (O 2 <0.3%) Stability of liposome dispersions in plastic bags
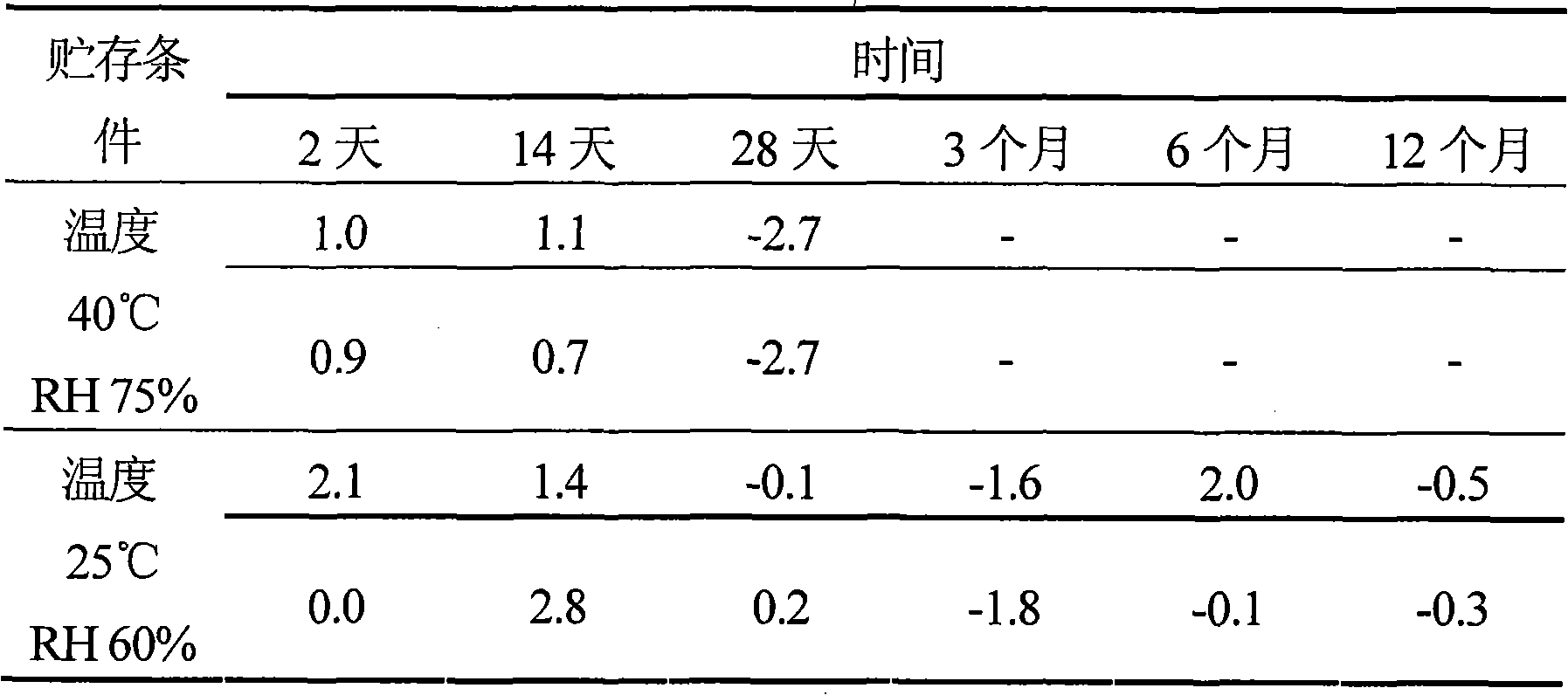
[0078] A 50 ml sample of the liposome dispersion containing 2.8 mM L-Cys prepared in Part II was packaged and sealed in a polypropylene (HFB tablet, Nipro Corporation) in an atmosphere with an oxygen concentration of less than 0.3%. ) into a rectangular (8 × 10 cm) bag. Packed bags further with oxygen absorber (AGELESS TM, Mitsubishi Gas Chemical Co., Ltd.) are packaged together in an oxygen-impermeable outer envelope (TECHBARRIER TM , Mitsubishi Plastics, Inc.). The resulting product was stored at a temperature of 40°C and a relative humidity of 75% or a temperature of 25°C and a relative humidity of 60%. Methemoglobin levels (Met%) were determined in duplicate (n=2) over time. The results are shown in Table 2 below.
[0079] Table 2.O 2 %Met of liposome-coated hemoglobin in plastic bags packed at < 0.3%
[0080]
[0081] As shown in Table 2, the % Met of the liposome di...
Embodiment 3
[0082] Example 3. Under hypoxia (O 2 <3%) Stability of liposome dispersions in plastic bags packed
[0083] Example 2 was repeated, except that the packaging operation was carried out in an atmosphere with an oxygen concentration of 3%. The results are shown in Table 3 below.
[0084] Table 3.O 2 %Met of liposome-coated hemoglobin in plastic bags packaged at <3%
[0085]
[0086] As shown in Table 3, the %Met of the liposome dispersion samples in the binary package did not increase when stored at 40°C, 75% RH for 1 month or at 25°C, 60% RH for 12 months.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com