Process for preparing 17-allyl amino geldanamycin (17-AAG) and other ansamycins
A technology of 17-AAG and angiomycin is applied in the field of preparing 17-allylaminogeldanamycin (17-AAG) and other ansamycins, and can solve the problems of instability, low yield, Low purity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
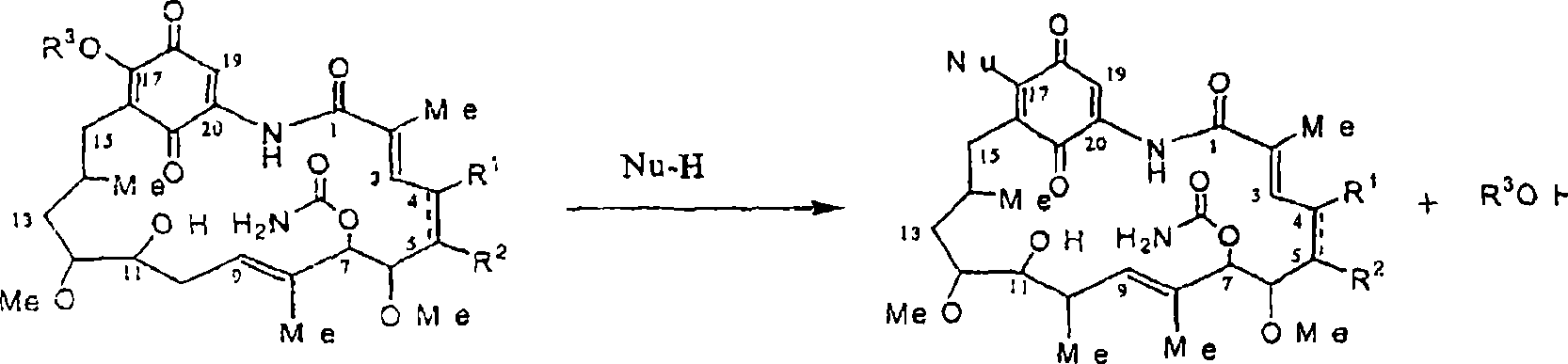
[0081] Synthesis of 17-allylaminogeldanamycin (17-AAG)
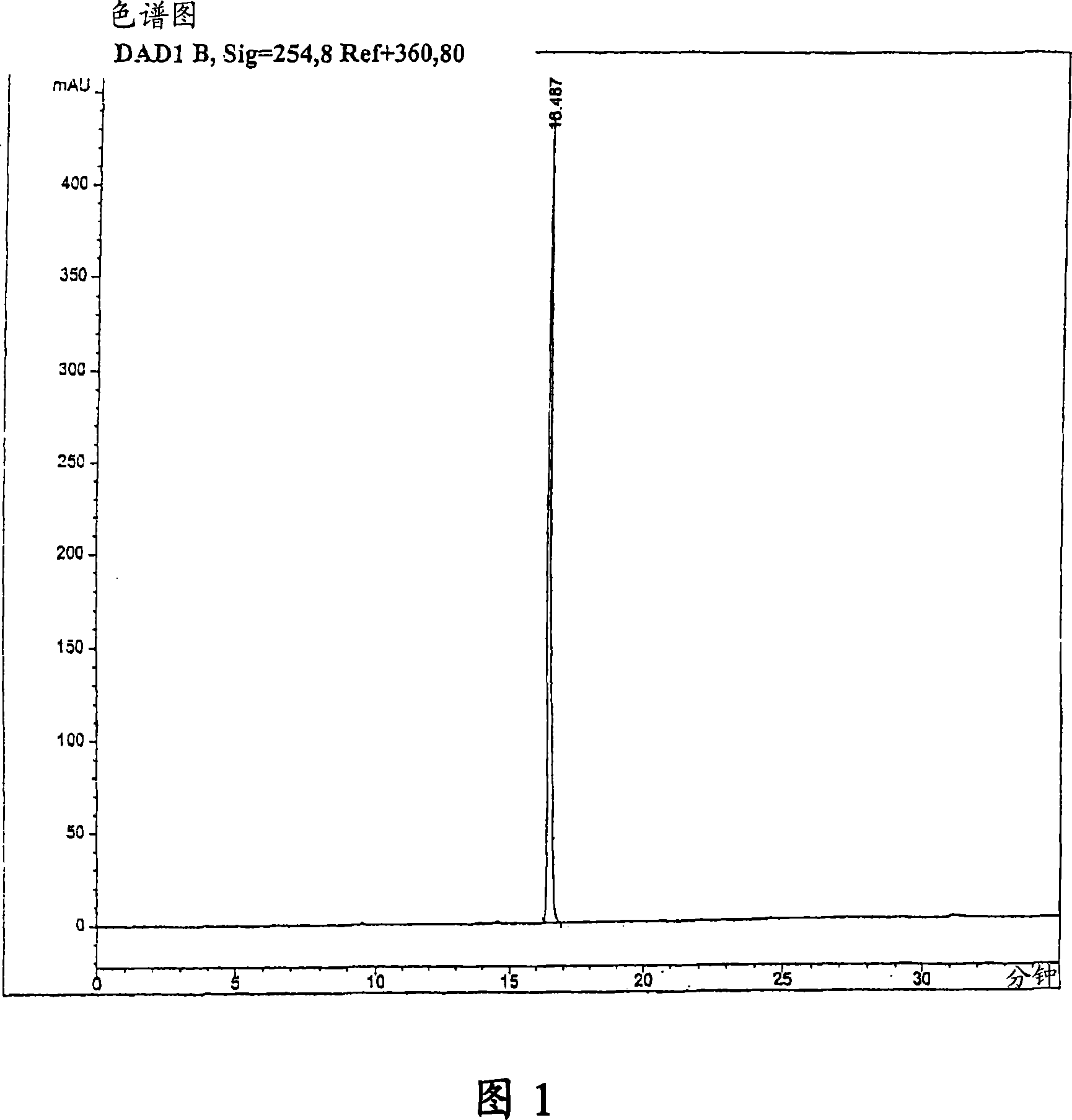
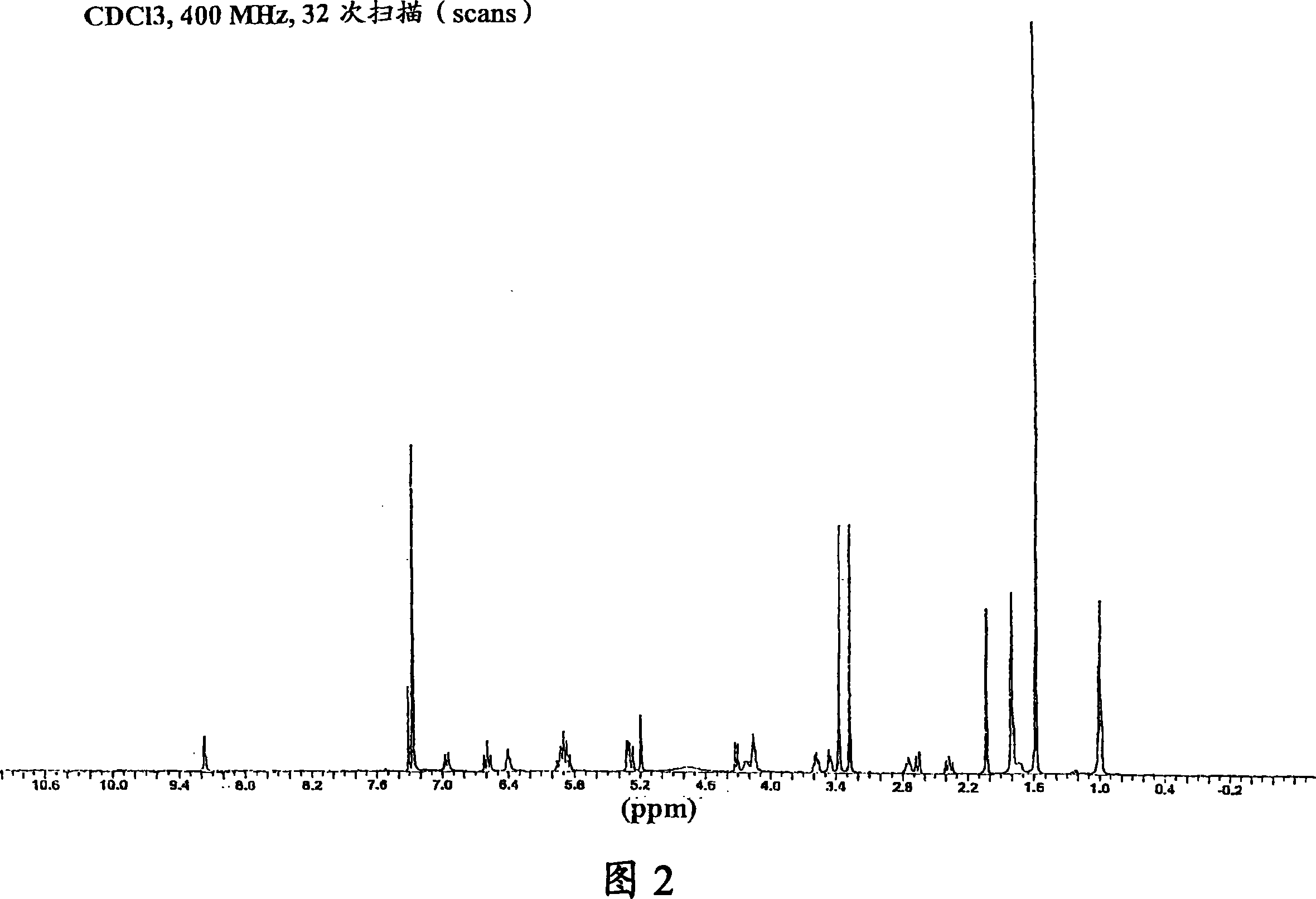
[0082] Put 45.0g (80.4mmol) of Geldanamycin in a 2L dry flask with 1.45L of dry THF, add 36.0mL (470mmol) of allylamine dropwise to it for more than 30 minutes, wherein the allylamine Place in 50 mL of dry THF. The reaction mixture was stirred at room temperature under nitrogen protection for 4 hours until TLC analysis showed that the reaction was complete [(GDM: bright yellow: Rf=0.40; (5%MeOH-95%CHCl 3 ); 17-AAG: purple: Rf = 0.42 (5% MeOH-95% CHCl 3 )]. The solvent was removed by rotary evaporation, and the crude product was mixed with 420 mL H at 25 °C 2 O:EtOH (90:10) was mixed, then filtered, and dried at 45 °C for 24 hours to obtain 40.9 g (66.4 mmol) of purple crystal 17-AAG (82.6% yield, purity >98% at 254 nm by HPLC monitoring) . MP 206-212°C. 1 The results obtained by H NMR and HPLC were consistent with that of the desired product.
Embodiment 2
[0084] 17-AAG crystallized in isopropanol
[0085] An alternative method of purification is instead crystallization in ethanol, but dissolving the crude product 17-AAG from Example 1 in 800 mL of 2-propanol (isopropanol) at 80°C or at reflux (about 82.2°C), and then Cool to room temperature. Filtration, followed by drying at 45° C. for 24 hours gave 44.6 g (72.36 mmol) of purple crystals of 17-AAG (90% yield, purity >99% at 254 nm by HPLC monitoring). MP 147-153°C. 1 The results obtained by H NMR and HPLC were consistent with that of the desired product.
Embodiment 3
[0087] Ethanol Washing of the 17-AAG Polymorph of Example 2
[0088] An optional method of purification is in 400 mL of H 2 The product 17-AAG in Example 2 was mixed in O:EtOH (90:10) at 25°C, filtered, and dried at 45°C for 24 hours to obtain 42.4 g (68.6 mmol) of purple crystals of 17-AAG (95% Yield, purity >99% at 254 nm monitored by HPLC). MP 147-153°C. 1 The results obtained by H NMR and HPLC were consistent with that of the desired product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com