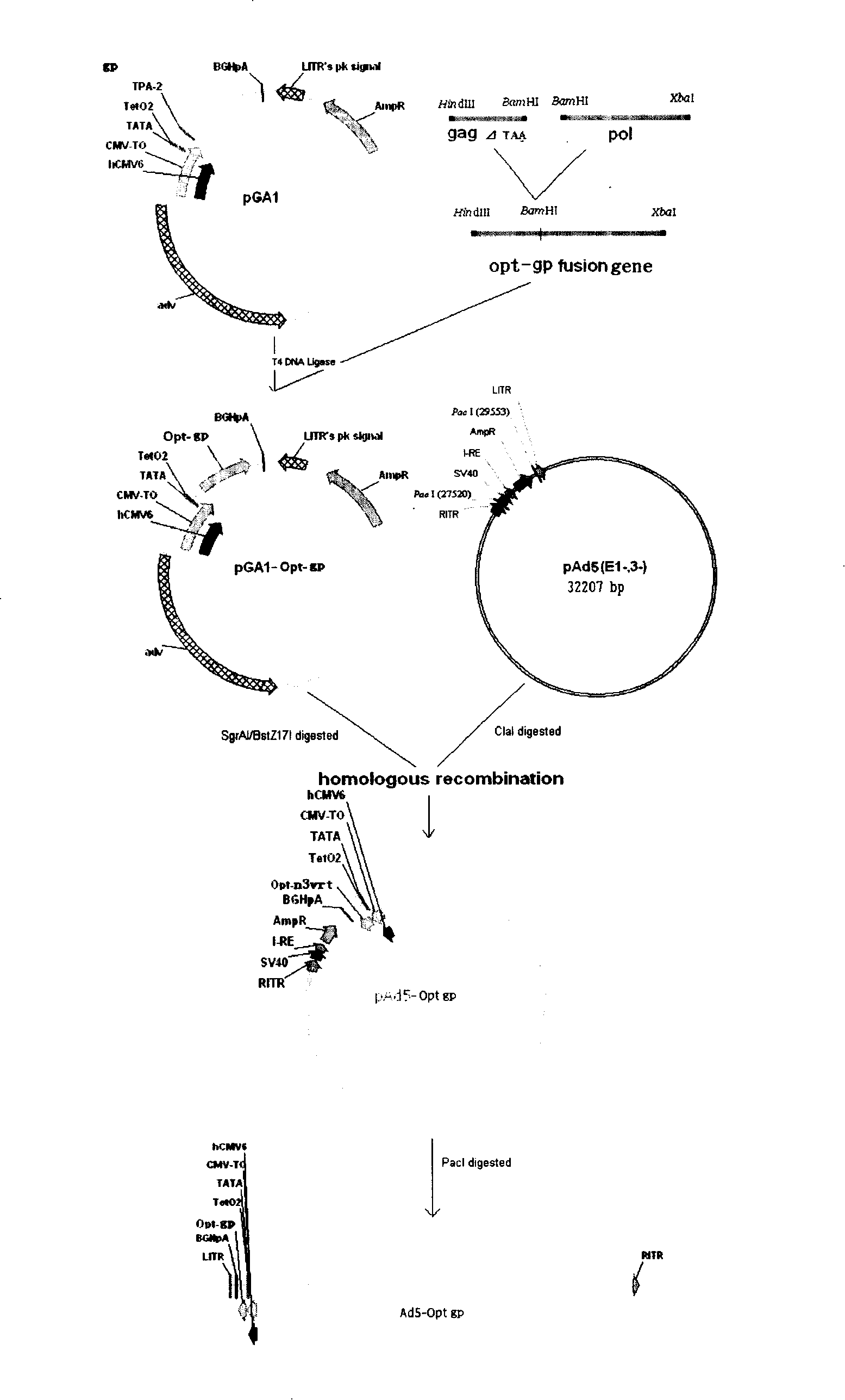
Adenovirus carrier vaccine carrying HIV gene
A carrier vaccine and adenovirus technology, applied in gene therapy, antiviral agents, genetic engineering, etc., can solve the problem of vaccine immunogenicity decline and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] HIV1 gag vaccine sequence:
[0053] 1. The 08BC gag consensus sequence is from lanl, and the sequence is
[0054] MGARASILRGGKLDKWEKIRLRPGGKKHYMLKHLVWASRELERFALNPGLLETSEGCKQIIKQLQPALQTGTEELRSLFNTVATLYCVHAEIEVRDTKEALDKIEEEQNKIQQKTQQAKEADEKVSQNYPIVQNLQGQMVHQPLSPRTLNAWVKVVEEKAFSPEVIPMFTALSEGATPQDLNTMLNTVGGHQAAMQMLKDTINEEAAEWDRLHPVHAGPVAPGQMREPRGSDIAGTTSTLQEQIGWMTNNPPIPVGEIYKRWIILGLNKIVRMYSPTSILDIKQGPKEPFRDYVDRFFKTLRAEQATQDVKNWMTDTLLVQNANPDCKTILRALGPGASLEEMMTACQGVGGPSHKARVLAEAMSQTNNTILMQRSNFKGSKRIVKCFNCGKEGHIAKNCRAPRKKGCWKCGKEGHQMKDCTERQANFLGKIWPSHKGRPGNFLQSRPEPTAPPAESFRFEETTPAPKQEPKDREPLTSLRSLFGSDPLSQ
[0055] 2. The following is the complete gag sequence of HIV1 (CRF08BC) containing optimized codons.
[0056]atgggcgccagggcctccatcctgaggggcggcaagctggacaagtgggagaagatcaggctgaggcctggcggcaagaagcactacatgctgaagcatctggtctgggcctccagggagctggagaggtttgccctgaaccctggcctgctggagacctctgagggctgcaagcagatcatcaagcagctgcagcctgccctgcagacaggcacagaggagctgaggtccctgttcaacacagtggccaccctgtactgtgtgcatg...
Embodiment 2
[0058] HIV1 pol vaccine sequence:
[0059] 1. The 08BC pol consensus sequence is from lanl, and the sequence is
[0060] FFREILAFPQGEAREFPPEQTRANSPTSRELQVRGDNPSSEAGTERQGTLNFPQITLWQRPLVSIKVGGQIKEALLDTGADDTVLEEVNLPGKWKPKMIGGIGGFIKVRQYEQIPIEICGKKAIGTVLVGPTPVNIIGRNMLTQLGCTLNFPISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALTAICDEMEKEGKITKIGPDNPYNTPIFAIRKKDSSKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKKKSVTVLDVGDAYFSVPLDKDFRKYTAFTIPSVNNETPGIRYQYNVLPQGWKGSPAIFQCSMTKILEPFRKQNPDIVIYQYMDDLYVGSDLEIGQHRTKIEELREHLLKWGFTTPDKKHQKEPPFLWMGYELHPDKWTVQPIQLPEKDSWTVNDIQKLVGKLNWASQIYPGIKVRQLCKLLRGAKALTDIVPLTEEAELELAENREILKEPVHGAYYDPSKELIAEIQKQGQDQWTYQIYQEPFKNLKTGKYAKMRTAHTNDVKQLTEAVQKIAMESIVIWGKIPKFRLPIQKETWETWWTDYWQATWIPEWEFVNTPPLVKLWYQLEKDPIAGVETFYVDGAANRETKIGKAGYVTDRGRKKIVSLTDTTNQKTELQAIYIALQDSGSEVNIVTDSQYALGIIQAQPDKSESELVNQIIEQLIKKERVYLSWVPAHKGIGGNEQVDKLVSNGIRKVLFLDGIDKAQEEHEKYHSNWRAMASDFNLPPIVAKEIVASCDQCQLKGEAMHGQVDCSPGIWQLDCTHLEGKIILVAVHVASGYIEAEVIPAETGQETAYFILKLAGRWPVKVIHTDNGSNFTSAAVKAACWWAGIQQEFGIPYNPQSQGVVESMNKELKKL...
Embodiment 3
[0076] HIV1 nef vaccine sequence:
[0077] 1. The consensus sequence of 08BC nef comes from lanl, and the sequence is
[0078] MGGKWSKSSIVGWPAIRERIRRTEPAADGVGAVSRDLEKHGAITSSNTADTNADCAWLETQEEEEVGFPVRPQVPLRPMTFKGALDLSFFLKEKGGLEGLIYSKKRQEILDLWVYHTQGYFPDWHNYTPGPGVRFPLTFGWCFKLVPVDPREVEEANEGEDNCLLHPVCQHGMEDEHREVLKWKFDHPSQLAKHRAREL
[0079] 2. The transformation steps are: Nef needs myristylation sites (the 2nd amino acid glycine G2, the 164th and 165th leucine Leu-164-Leu-165) to exert most of the functions, these three sites Both mutated to alanine, Nef is functionally inactive.
[0080] 3. The following is the complete nef sequence of HIV1 (CRF08BC) containing optimized codons.
[0081] Atggctggcaagtggtccaagtcctccattgtgggctggcctgccatcagggagaggatcaggaggacagagcctgctgctgatggcgtgggcgctgtctccagggacctggagaagcatggcgccatcacctcctccaacacagctgacaccaatgctgactgtgcctggctggagacccaggaggaggaggaggtgggcttccctgtgaggccccaggtgcccctgaggcccatgaccttcaagggcgccctggacctgtccttcttcctgaaggagaagggcggcctggagggc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com