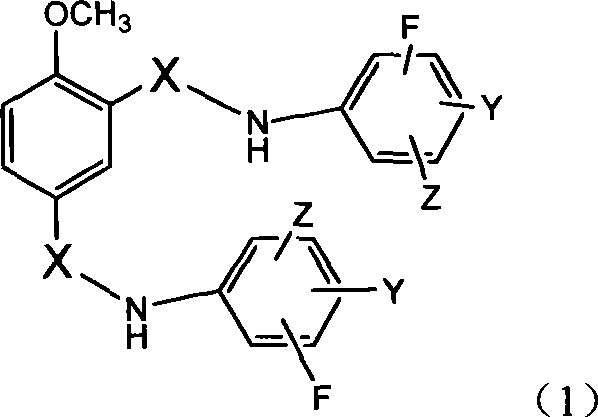
N,N'-difluorophenyl derivative of 4-methoxyl-1,3-phthalamide and use thereof
A technology of difluorophenyl and phthalamide, which is applied in the field of anti-platelet aggregation and anti-inflammatory drugs, and can solve problems that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0473] Example 1: 4-methoxy-N, N'-bis-(3,5-difluorophenyl)-1,3-benzenedicarboxamide (C 21 h 14 f 4 N 2 o 3 )Synthesis
[0474] Dissolve 1.3g (10.1mmol) of 3,5-difluoroaniline in 15mL of tetrahydrofuran, add it to a 100mL round bottom flask, and dissolve 1.0g (4.3mmol) of 4-methoxy-1,3-phthaloyl chloride React in 10mL tetrahydrofuran for 6 hours. The solvent was evaporated. The solid was successively recrystallized from ethanol to obtain 1.2 g of white needle-like crystals. Yield: 66.7%, mp: 248-250°C.
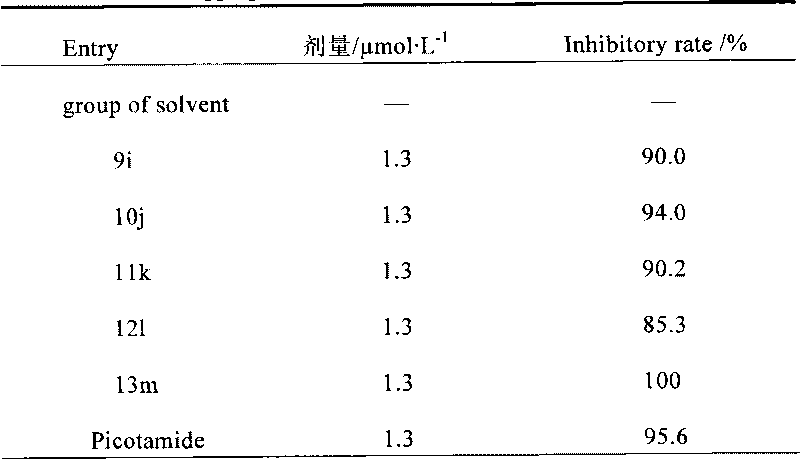
[0475] The group number of the compound in the rabbit platelet aggregation reaction experiment is 9i.
[0476] Infrared spectroscopy (IR) and nuclear magnetic resonance spectroscopy ( 1 H-NMR) confirmed the structure of 9i: IR(KBr)cm -1 : 3362.5, 3102.9 (υ NH ), 1685.7, 1668.5 (υ C=O ), 1607.9, 1559.7, 1495.9, 1431.1 (υ C=C ), 1311.3(υ C-N ), 1266.6, 1020.2 (υ C-O-C ), 1101.7(υ CF ), 843.6, 828.1, 669.5 (υ CH ); 1 H-NMR (Acetone) δ (ppm): 4.18 (s, 3H, -OCH 3 ...
Embodiment 2
[0477] Example 2: 4-methoxy-N, N'-bis(3,5-difluorophenyl)-1,3-benzenedisulfonamide (C 19 h 14 f 4 N 2 o 5 S 2 )Synthesis
[0478] Dissolve 1.0g (7.8mmol) of 3,5-difluoroaniline in 10mL of tetrahydrofuran, add it to a 100mL round bottom flask, add 1.0g (3.3mmol) of 4-methoxy-1,3-benzenedisulfonyl chloride React in the above-mentioned reaction bottle for 6 hours. The solvent was evaporated. The solid was recrystallized from ethanol to obtain 1.0 g of white crystals. Yield: 61.7%, mp: 212-214°C.
[0479] The group number of the compound in the rabbit platelet aggregation reaction experiment is 10j.
[0480] Infrared spectroscopy (IR) and nuclear magnetic resonance spectroscopy ( 1 H-NMR) confirmed the structure of 10j: IR (KBr) cm -1 : 3256.6, 3114.4 (υ NH ), 2953.0 (υ CH3 ), 1626.1, 1488.4, 1438.5, 1410.1 (υ C=C ), 1285.8, 1064.1 (υ C-O-C ), 1350.9, 1185.3, 1163.3 (υ SO2 ), 1309.4(υ C-N ), 993.3(υ CF ), 892.8, 838.8, 708.7 (υ CH ); 1 H-NMR (Acetone) δ (ppm): ...
Embodiment 3
[0481] Example 3: 4-methoxy-N, N'-bis(3-trifluoromethyl-4-fluorophenyl)-1,3-benzenedicarboxamide (C 23 h 14 f 8 N 2 o 3 )Synthesis
[0482] Dissolve 1.0g (5.6mmol) of 3-trifluoromethyl-4-fluoroaniline in 10mL of tetrahydrofuran into a 100mL round-bottomed flask, and add 0.6g (2.6mmol) of 4-methoxy-1,3-benzenedimethoxy The acid chloride was dissolved in 10 mL of tetrahydrofuran and added to the above reaction flask to react for 6 hours. The solvent was evaporated. The solid was recrystallized from ethanol to obtain 1.0 g of taupe solid. Yield: 75.2%, mp: 224-226°C.
[0483] The group number of the compound in the rabbit platelet aggregation reaction experiment is 11k.
[0484] Infrared spectroscopy (IR) and nuclear magnetic resonance spectroscopy ( 1 H-NMR) confirmed the 11k structure: IR (KBr) cm -1 : 3357.0(υ NH), 1675.4, 1654.7 (υ C=o ), 1552.0, 1505.3, 1427.2 (υ C=C ), 1329.4(υ CF3 ), 1270.1(υ C-N ), 1250.0(υ C-N ), 1054.2(υ C-O-C ), 1125.0(υ CF ); 1 H-NM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com