Recombination expression carrier and method for soluble expressing human I-type metallothionin
A metallothionein and expression vector technology, which is applied in the field of expressing and producing human type I metallothionein in a soluble form, and a recombinant expression vector for expressing human type I metallothionein, can solve the problem of expensive protease cost, time-consuming, and inability to obtain MT production and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] Example 1: Artificial synthesis of small molecule ubiquitin-related modifier mature peptide and human type I metallothionein fusion protein gene
[0123] Thirteen oligonucleotide chains must be synthesized (synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd.) , respectively: SUMO-F1 (SEQ ID No: 11), SUMO-F2 (SEQ ID No: 12), SUMO-F3 (SEQ ID No: 13), SUMO-F4 (SEQ ID No: 14), SUMO- R1 (SEQ ID No: 15), SUMO-R2 (SEQ ID No: 16), SUMO-R3 (SEQ ID No: 17), SUMO-R4 (SEQ ID No: 18), MT-F1 (SEQ ID No: 19), MT-F2 (SEQ ID No: 20), MT-R1 (SEQ ID No: 21), MT-R2 (SEQ ID No: 22) and MT-R3 (SEQ ID No: 23). The specific process of synthesis is shown in Figure 3. Briefly, first, in the first step of PCR reaction, 2 μl (10 μmol L -1 ), dNTP (each 2.5μmol·L -1 ) 10 μl, 10 μl of 10x PCR pfu buffer, add water to a total volume of 100 μl. After denaturation at 94°C for 5 minutes, slowly lower the temperature to 55°C, then add pfuDNA polymerase, then extend at 72°C for...
Embodiment 2
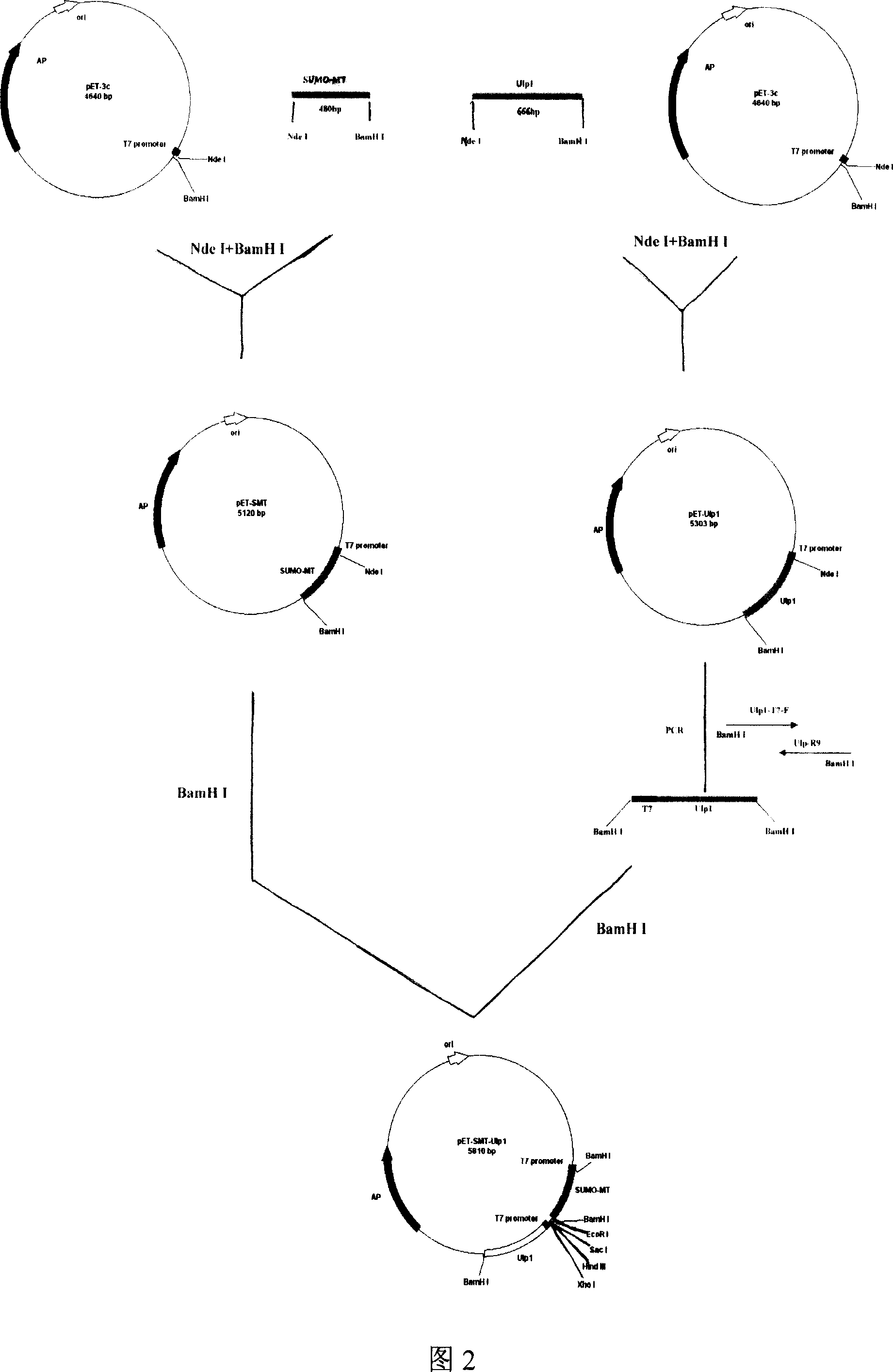
[0124] Embodiment 2: Construction of recombinant vector pET-Ulp1
[0125] First synthesize 18 oligonucleotide chains (synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd.), respectively: Ulp-F1 (SEQ ID No: 32), Ulp-F2 (SEQ ID No: 33), Ulp -F3 (SEQ ID No: 34), Ulp-F4 (SEQ ID No: 35), Ulp-F5 (SEQ ID No: 36), Ulp-F6 (SEQ ID No: 37), Ulp-F7 (SEQ ID No : 38), Ulp-F8 (SEQ ID No: 39), Ulp-F9 (SEQ ID No: 40), Ulp-R1 (SEQ ID No: 41), Ulp-R2 (SEQ ID No: 42), Ulp-R3 (SEQ ID No: 43), Ulp-R4 (SEQ ID No: 44), Ulp-R5 (SEQ ID No: 45), Ulp-R6 (SEQ ID No: 46), Ulp-R7 (SEQ ID No: 47 ), Ulp-R8 (SEQ ID No: 48) and Ulp-R9 (SEQ ID No: 49). According to the method described in Example 1 and the steps in Figure 4, three large fragments were artificially synthesized using PCR technology, named respectively as UI (SEQ ID No: 50), UII (SEQ ID No: 51) and UIII (SEQ ID No: 52). Then, using UI and UII as common templates, adding Ulp-F6 and Ulp-R3 as upstream and downstream primers,...
Embodiment 3
[0126] Embodiment 3: Construction of recombinant expression vector pET-SMT-Ulp1
[0127] In order to construct a cDNA sequence containing a mature peptide encoding a small molecule ubiquitin-related modification factor under the control of the T7 promoter and a human type I metallothionein fusion protein cDNA sequence connected in tandem under the control of the T7 promoter and under the control of the T7 promoter The recombinant expression vector of the ubiquitin-related modifier protease 1 gene, first, synthesize an oligonucleotide primer Ulp1-T7-F based on the T7 promoter: 5'CT GGA TCC GAA TTC GAG CTC AAG CTT CTC GAGTAA TAC GAC TCA CTA TAG GGA G3' (synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd.; SEQ ID NO: 54). The 5' end of this primer contains a multiple cloning site. According to the conventional PCR method, use the recombinant expression vector pET-Ulp1 as the template, Ulp1-T7-F and Ulp-R9 as the upstream and downstream primers, and enter t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com