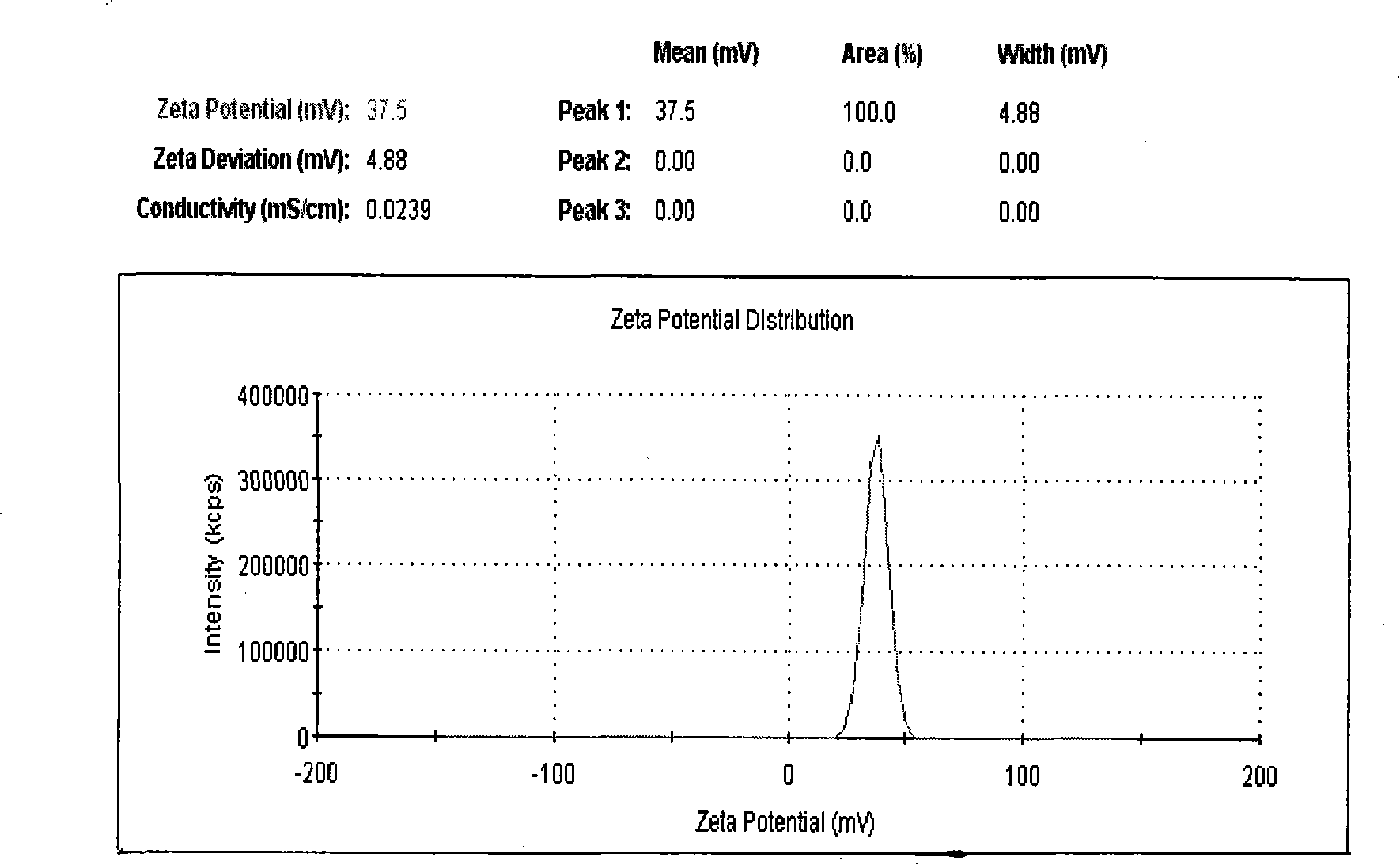
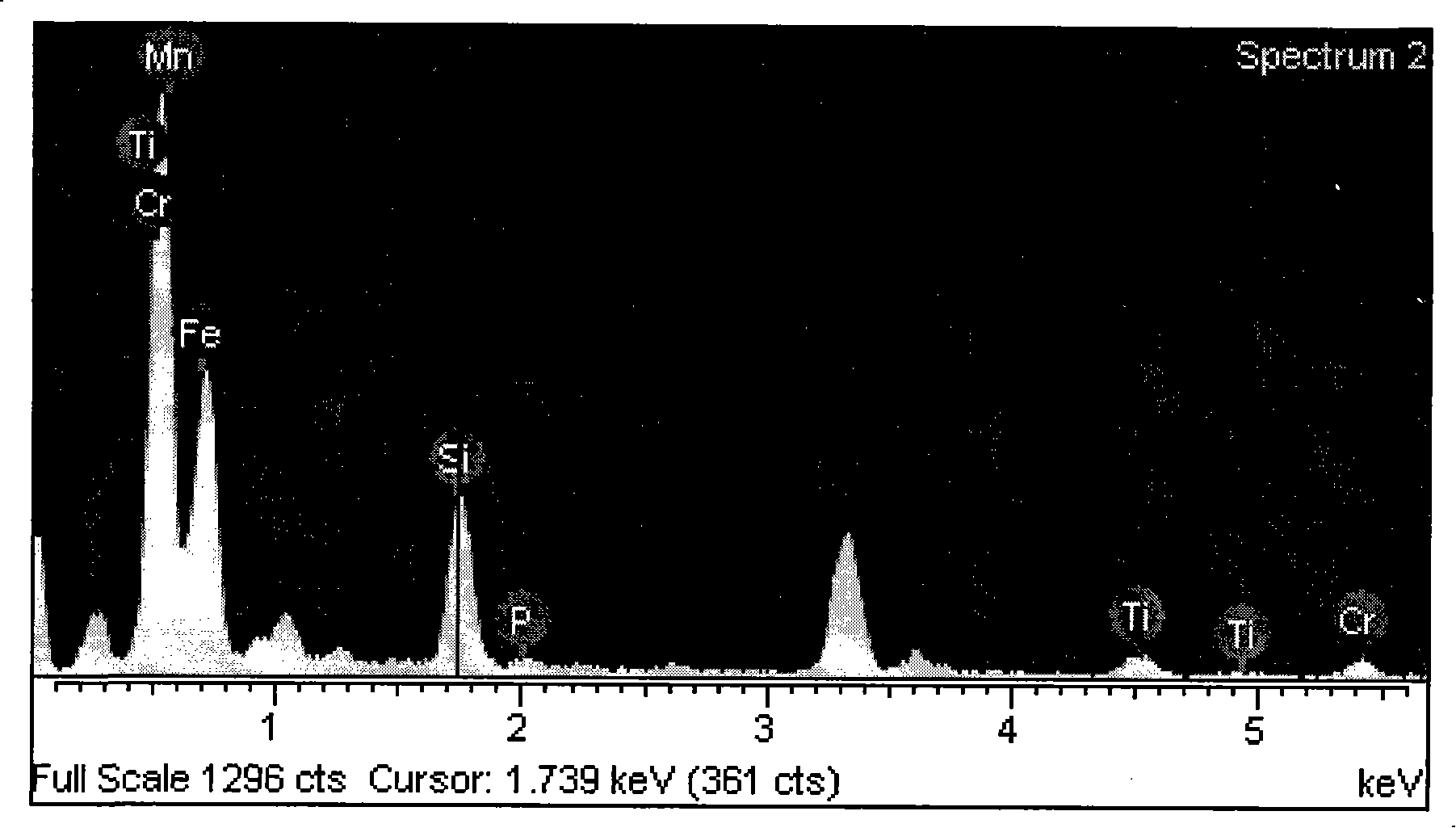
Target preparation consisting of liposome and nucleic acid coating contrast agent
A technology of contrast agent and liposome, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1 Preparation of magnetically targeted liposomes
[0034] 1.1 Accurately weigh 20 mg of dioleoylphosphatidylethanolamine (DOPE, Fluka Company), 3β-[N-(N', N'-dimethylaminoethyl)] carbamoyl-cholesterol (DC-Chol, Sigma Company) 20mg, cholesterol (Chol, Japan Wako Pure Chemical Industry Co., Ltd.) 5mg and α-tocopherol (Shanghai No. 2 Pharmaceutical Factory) 1mg were placed in a 250ml round bottom flask, and 8ml of chloroform was added to dissolve it completely.
[0035] 1.2 Dilute ferric oxide tartaric acid solution with water for injection to 5 mg / ml in a cell pulverizer (JY92-IIN, Ningbo Xinzhi Biotechnology Co., Ltd.) and ultrasonically (400w, 5s each time, 40 times) to disperse Evenly, take 1.5ml and put it into the above round-bottomed flask, and ultrasonically (500w) for 30min in a water bath ultrasonic cleaner (SK5200LH, Shanghai Kedao Ultrasonic Instrument Co., Ltd.) to form a stable W / O emulsion.
[0036] 1.3 Put the organic solvent on a rotary evaporator (N-1001...
Embodiment 2
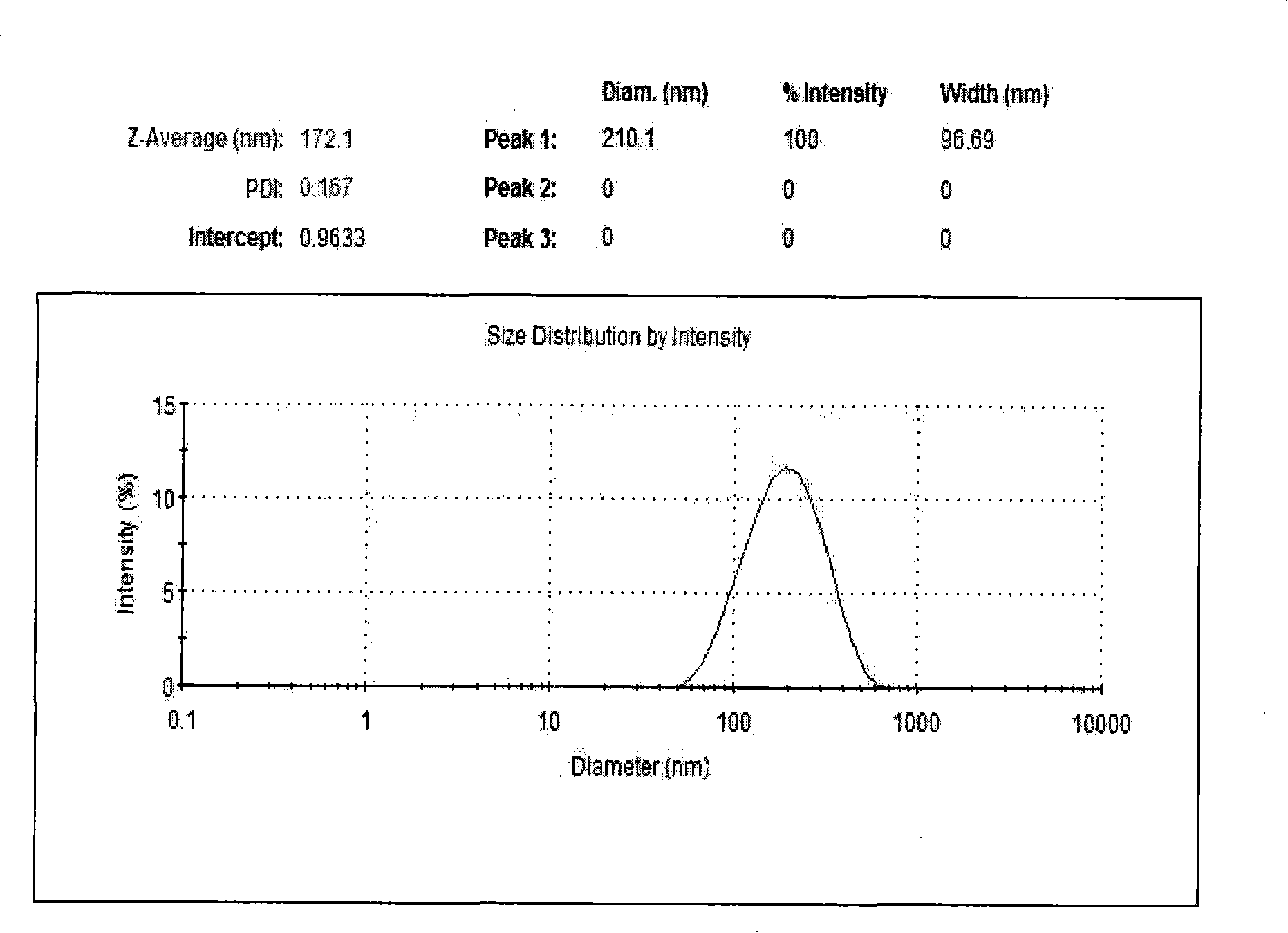
[0046] 1.5 Homogenize the prepared liposome suspension through a high-pressure homogenizer (Em-C3, Avestin, Canada) (15000psi, 3 cycles), and the obtained positive liposome particle size distribution of the encapsulating contrast agent 100-200nm.
[0047] All the other steps are the same as in Example 1.
Embodiment 3
[0049] 1.1 Accurately weigh 20 mg of dipalmitoylphosphatidylethanolamine (DOPC, NOF Corporation), 30 mg of 3β-[N-(N', N'-dimethylaminoethyl)] carbamoyl-cholesterol (DC-Chol) Add 8ml of chloroform / diethyl ether (4, V / V) to a 250ml round bottom flask to dissolve it completely.
[0050] 1.2 Dilute ferric oxide tartaric acid solution with water for injection to 3 mg / ml in a cell pulverizer and sonicate (400w, 5s each time, 40 times) to make it evenly dispersed, take 1.5ml into the above round bottom flask, and ultrasonicate in a water bath 30min to form a stable W / O emulsion.
[0051] 1.4 Add 10ml of phosphate buffer (pH 7.4) and vortex to make the gel fall off. Put it on the rotary evaporator and continue rotary evaporation for 2 hours until the liposome suspension is formed.
[0052] All the other steps are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com