Inorganic selenium for treatment of cancer
A cancer, selenate technology, applied in drug combinations, organic active ingredients, sulfur/selenium/tellurium active ingredients, etc., can solve problems such as differences in tumor progression of human prostate tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
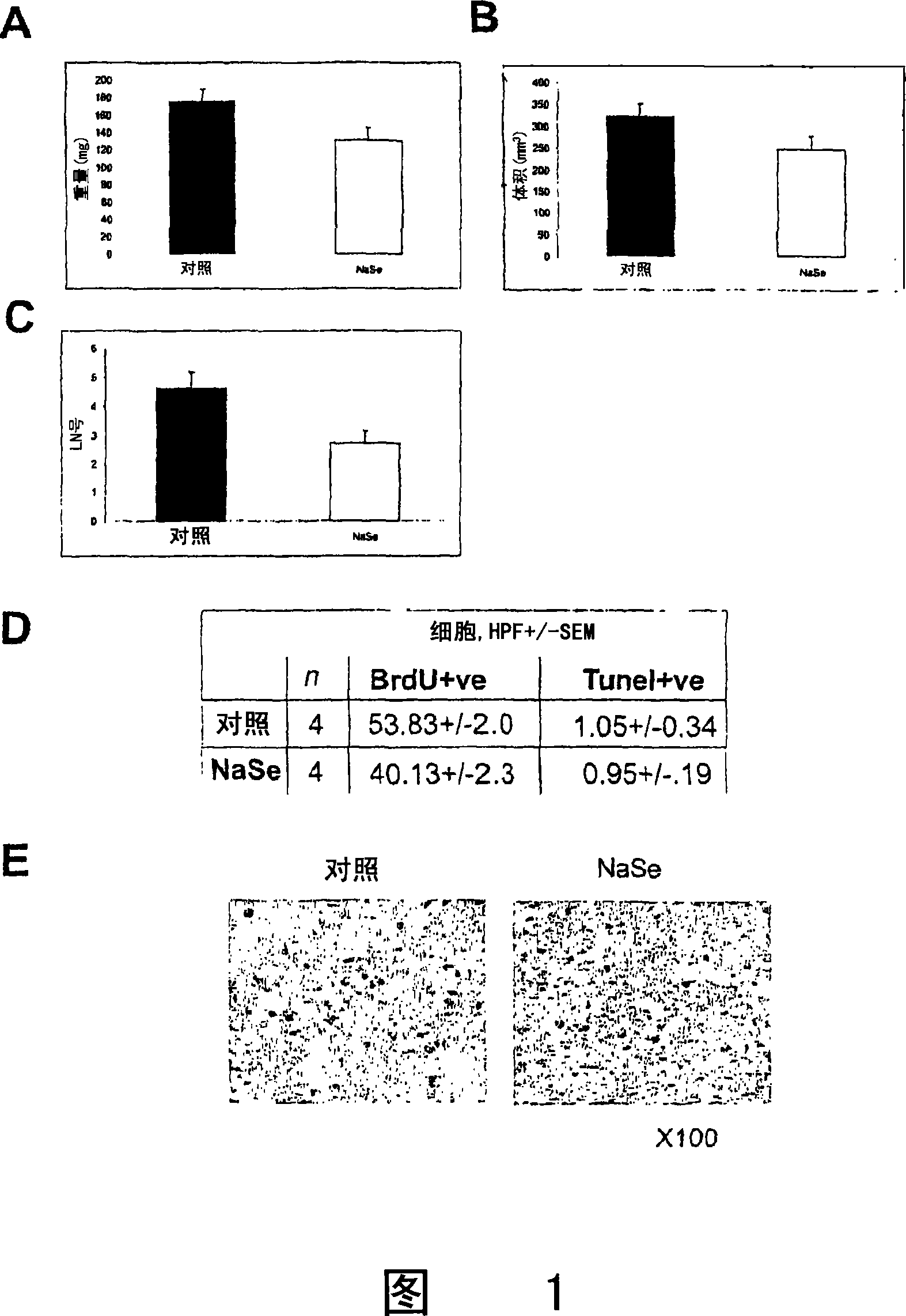
[0105] Inhibition of prostate tumor progression with sodium selenate through inhibition of protein kinase B / AKT activation
[0106] Materials and methods
[0107] cell culture
[0108] Cell culture experiments included the PC-3 cell line obtained from the American Type Culture Collection (Manassas, Virginia, USA). Cells are typically cultured with RPMI 1641 (Invitrogen) supplemented with 10% fetal bovine serum and 1% antibiotic / antimycotic cocktail (Invitrogen). At 37°C, 5% CO 2 maintain cells. Sodium selenate and selenomethionine (Sigma) were prepared into a 10 mM stock solution with distilled water, sterilized by filtration, and then diluted with culture medium for in vitro experiments.
[0109] Animal experiment
[0110] Animal experiments included the use of BALB / c nude mice. On the first day of the experiment, 6-week-old BALB / c nude mice were anesthetized with an intraperitoneal injection of ketamine 100 mg / kg and xylazine 20 mg / kg. Under a magnifying glass, ...
Embodiment 2
[0154] Reduced prostate tumor growth due to synergistic effect of sodium selenate and paclitaxel
[0155] Materials and methods
[0156] Will 1×10 6 Prostate tumor PC-3 cells were injected into the prostate of nude mice. After 3 days, 5 ppm of selenium in the form of sodium selenate was given to mice receiving selenate in the drinking water. Paclitaxel at 10 mg / kg in 10% Cremophor EL / 25% ethanol / 65% PBS solution was administered intraperitoneally to animals receiving paclitaxel once a week. 10 animals were used in each group. Five weeks after the injection, the animals were sacrificed, the prostates were removed, the attachments were dissected, and the tumors were weighed. Lymph node lesions in the retroperitoneal cavity were explored, and lymph nodes with a diameter greater than 0.5 mm were removed. The tumor was then sectioned in multiple layers at 50 μm slices, using the standard volumetric formula (a+b 2 / 2) Calculation of tumor volume from digitized images.
[01...
Embodiment 3
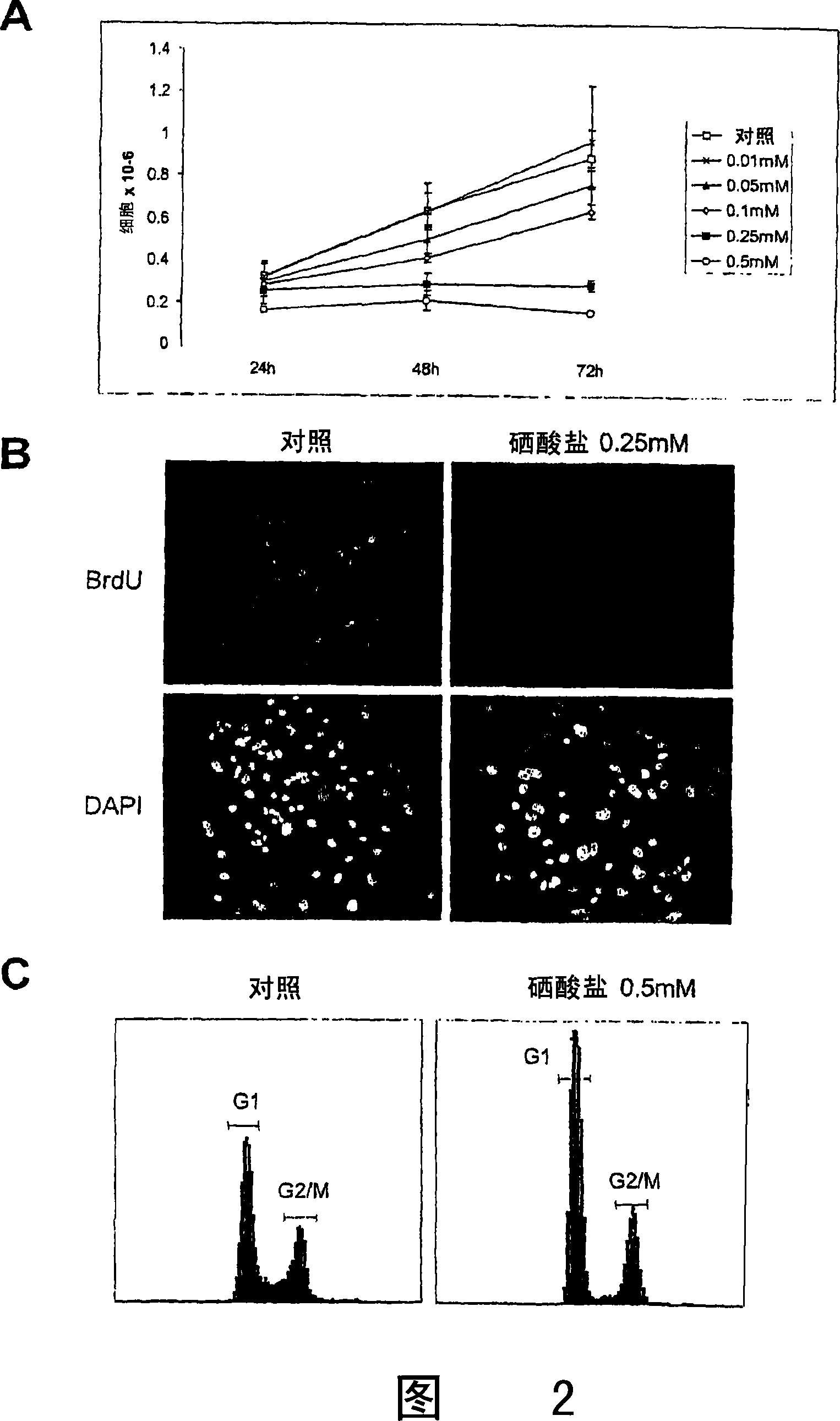
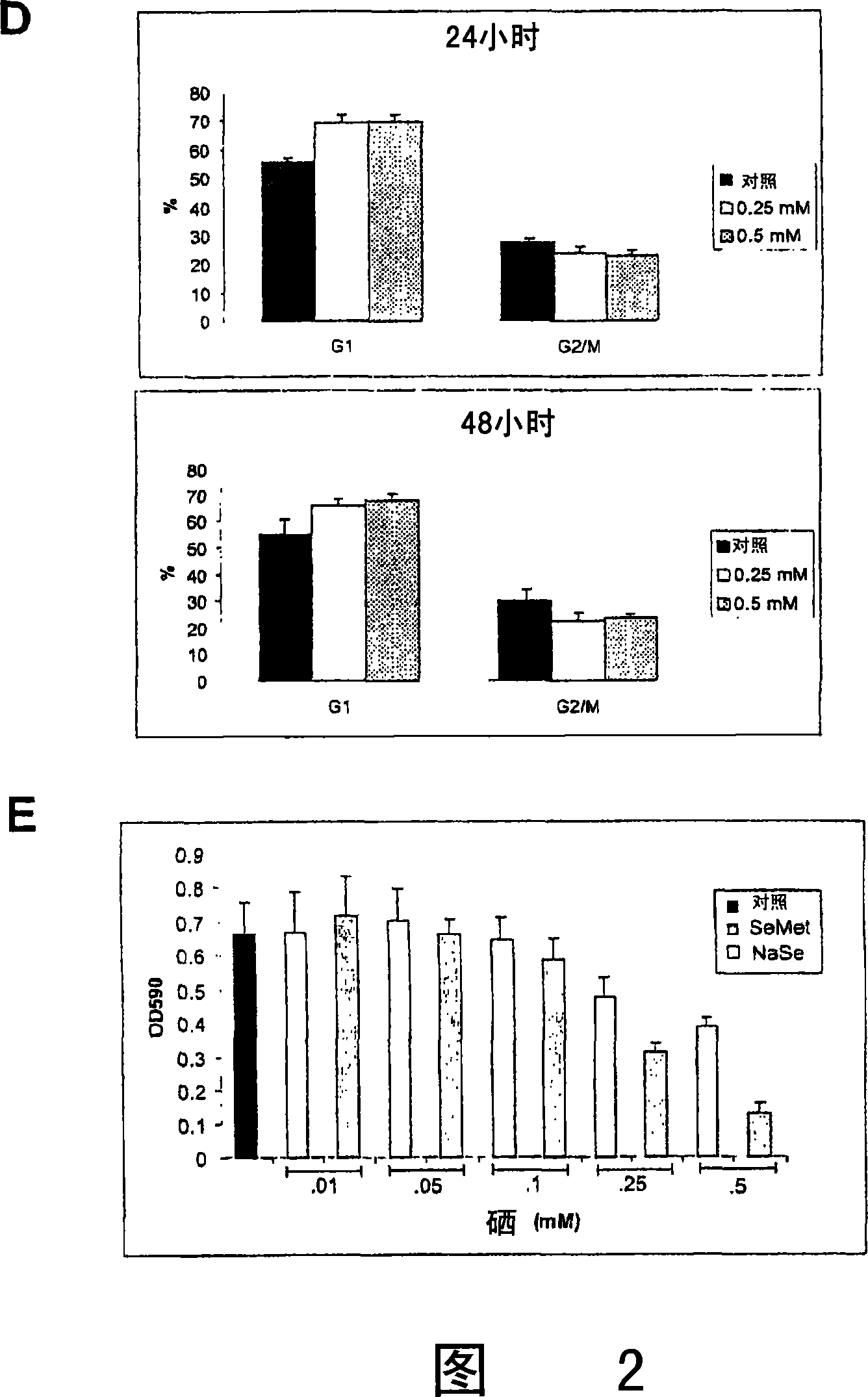
[0164] Comparison Test Between Selenate and Selenite
[0165] Materials and methods
[0166] Cytotoxic
[0167] Cytotoxicity after treatment with 5 μM or 50 μM sodium selenate or sodium selenite involves determination of cytotoxicity by trypan blue exclusion. will be 5×10 3 Human prostate cancer PC-3 cells were seeded in 24-well plates and treated with 5 μM or 50 μM selenate or selenite 8 hours later. A dose range of 5 μM or 50 μM is equivalent to 0.1 mg / kg-1.25 mg / kg. Cells were harvested 24, 48, 72, and 96 hours after the addition of selenate or selenite, and the percent viable cells were assessed by staining with trypan blue. The results are shown in Figure 9, which depicts the mean and SD of three independent experiments. Figure 9 shows that selenate is a cytostatic substance, while similar concentrations of selenite are cytotoxic. Therefore, selenite is not suitable for combination therapy in animals.
[0168] To differentiate the relative cytotoxic effects of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com