Truncated LHRH formulations
A domain, lipopeptide technology applied in the field of truncated LHRH formulations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Each group of dogs received a total of 5n moles of a mixed peptide consisting of P25, P35 and P62 coupled to LHRH(2-10) as the first dose. As a second and third dose, one group of dogs received the same vaccine, while a second group received a vaccine consisting only of P25 coupled to LHRH(6-10). Dogs received three doses of the vaccine at 0, 4, and 14 weeks.
[0078] Vaccine preparation: 1 to 1.5 mg of each peptide was weighed and dissolved in 100 ul of 4M urea. Solutions corresponding to 1.7 nmoles of each peptide were mixed and diluted with isotonic saline to the prescribed dose volume for injection (1 ml per dose). Add 150ug of Iscomatrix to each dose (CSL, Melbourne, Australia) as an adjuvant. The vaccine is given to the dog on the back of the neck.
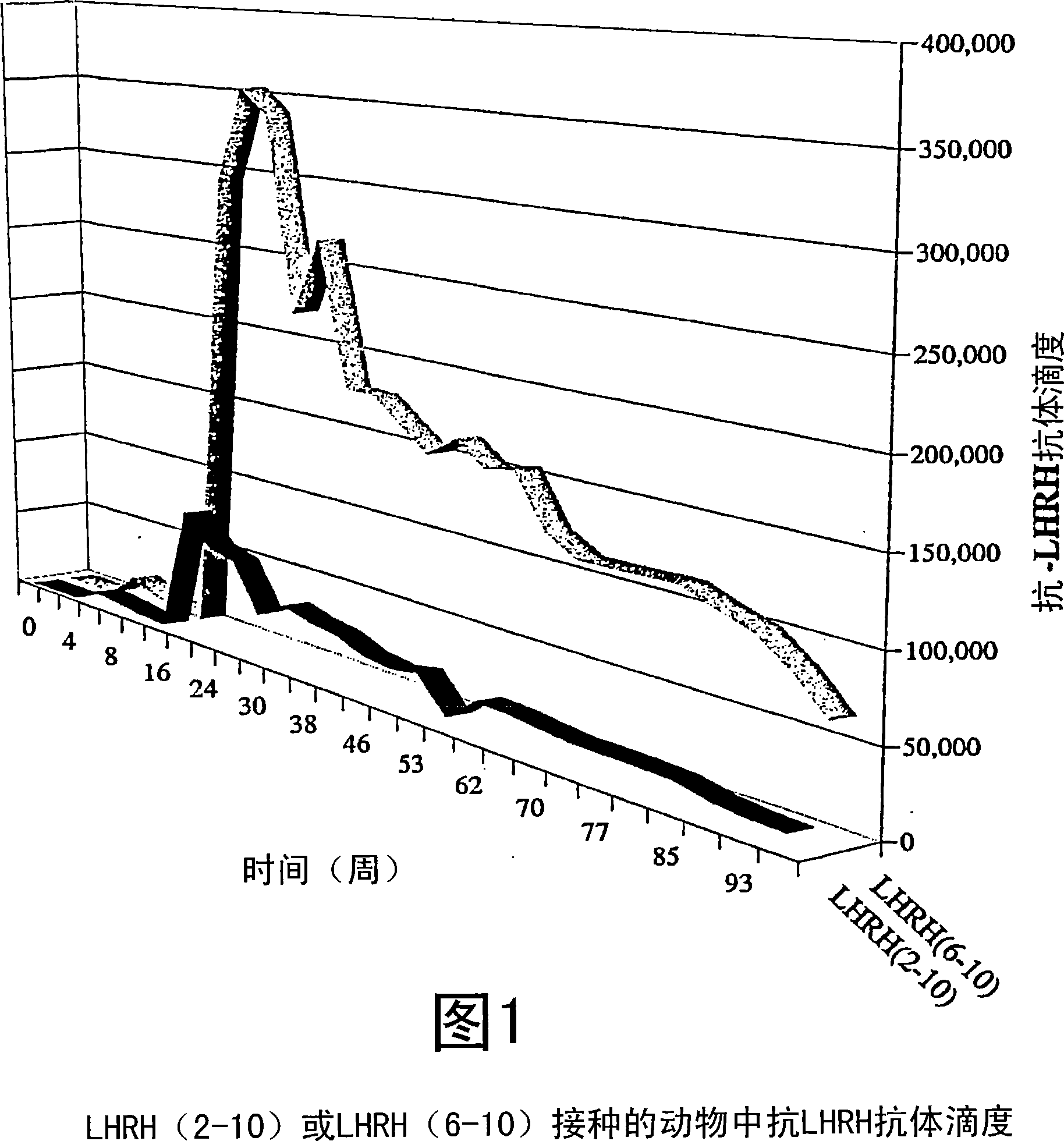
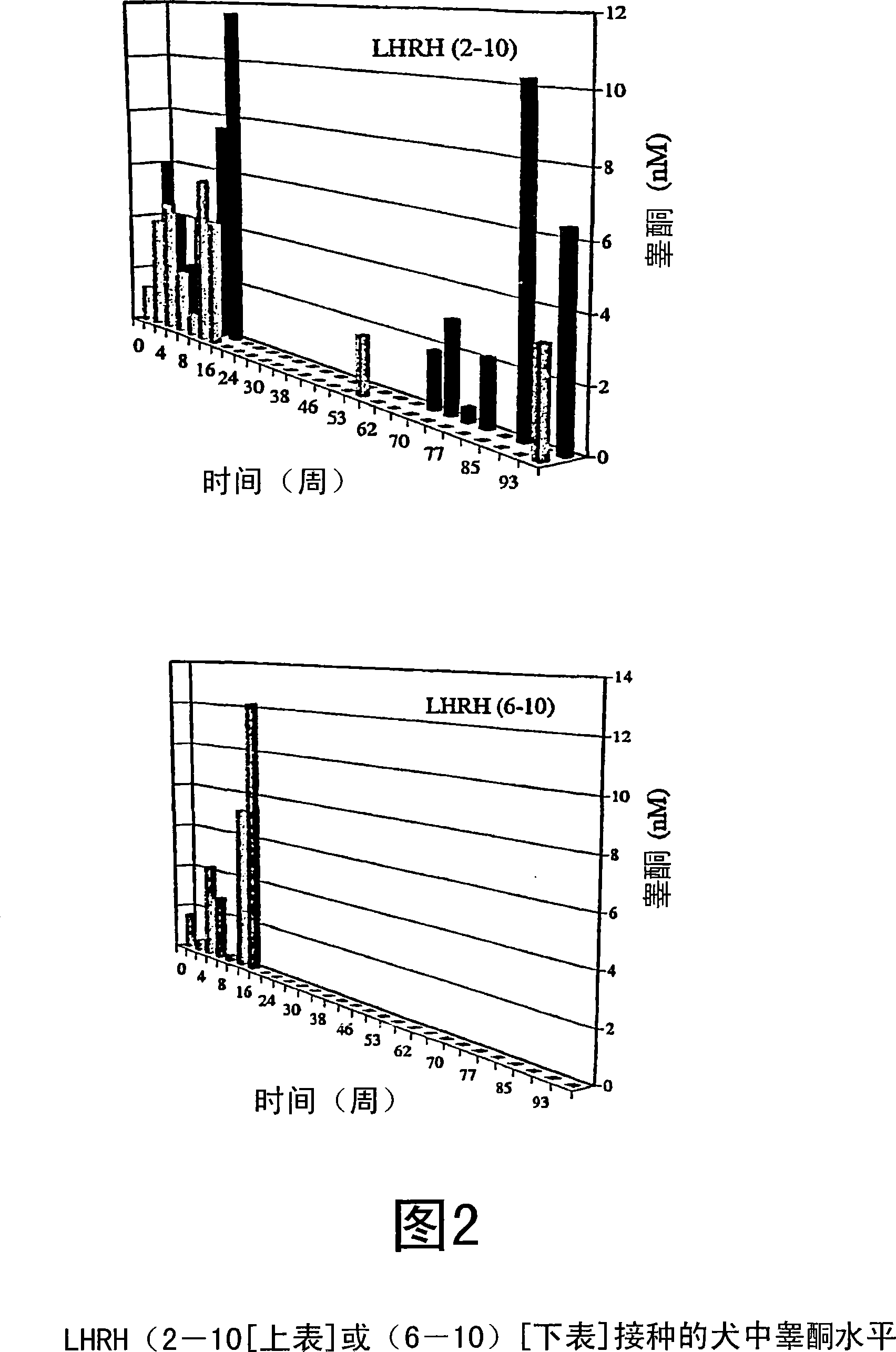
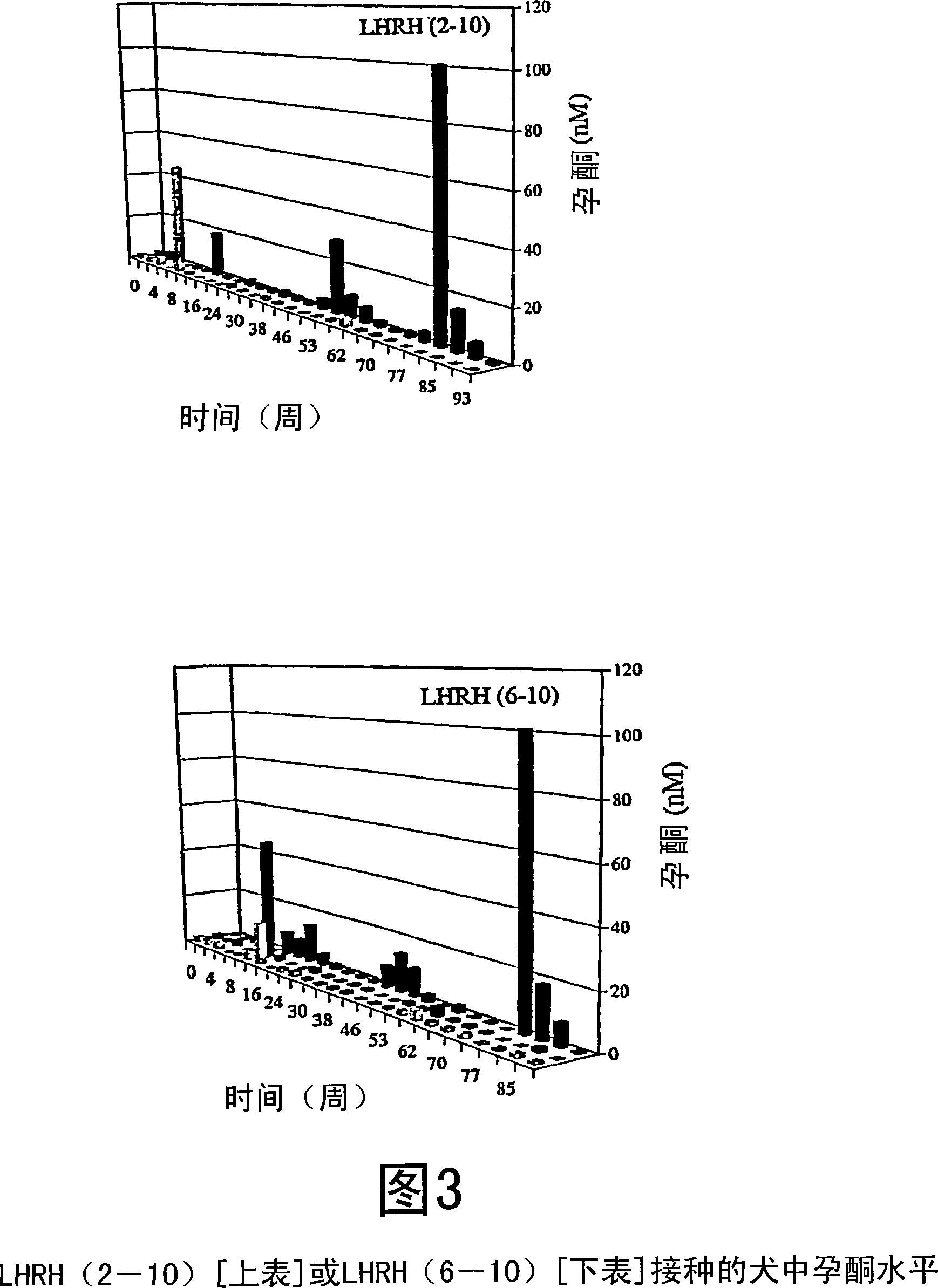
[0079] The measured antibody titers are shown in Figure 1. Simultaneous testosterone and progesterone levels are shown in Figure 2 and Figure 3, respectively.
Embodiment 2
[0081] Vaccination regimen:
[0082] The Beagle / foxhounds were divided into three groups. One group (12 dogs) received three doses of 5n molar total peptide consisting of a mixture of p25, p35 and p62 peptides coupled to LHRH (6-10).
[0083] A second group (6 dogs) received three doses of 5n molar total peptide consisting of a mixture of p25, p35 and p62 peptides coupled to LHRH (2-10).
[0084] A third group (6 dogs) received three doses of 5n molar total peptide consisting of a mixture of p25, p35 and p62 peptides coupled to LHRH (2-10) as the first dose. For the second and third doses, the vaccine consisted of a mixture of the same peptides conjugated to LHRH(6-10).
[0085] Dogs received three doses of the vaccine at 0, 4, and 14 weeks.
[0086] vaccine preparation
[0087] Weigh 1 to 1.5 mg of each peptide and dissolve in 100 ul of 4M urea respectively. Solutions corresponding to 1.7 nmoles of each peptide were mixed and diluted with isotonic saline to the prescribe...
Embodiment 3
[0091] Vaccination regimen:
[0092]Divide the Beagles / Foxhounds into two groups. The first group (15 dogs) received three doses of 35n molar total peptide consisting of a mixture of p4, p10, p24, p25, p27, p35 and p62 peptides coupled to LHRH (6-10).
[0093] A second group (15 dogs) received three doses of 35n molar total peptide consisting of a mixture of p4, p10, p24, p25, p27, p35 and p62 peptides coupled to LHRH (4-10).
[0094] Dogs received three doses of the vaccine at 0, 4, and 14 weeks.
[0095] Vaccine preparation:
[0096] Weigh 1 to 1.5 mg of each peptide and dissolve in 100 ul of 4M urea respectively. Solutions corresponding to 5 nmoles of each peptide were mixed and diluted with isotonic saline to the prescribed dose volume for injection (1 ml per dose). Add 150ug of Iscomatrix to each dose (CSL, Melbourne, Australia) as an adjuvant. The vaccine is given to the dog on the back of the neck.
[0097] The experimental results are shown in Figure 11 to Fig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com