Production of injecting reductive glutathione
A technology of glutathione and glutathione sodium, which is applied in the direction of drug combination, antidote, digestive system, etc., can solve the problems of easy oxidation of reduced glutathione, easy excess of water and related substances, etc., reaching a high level Uniformity, stable product quality, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
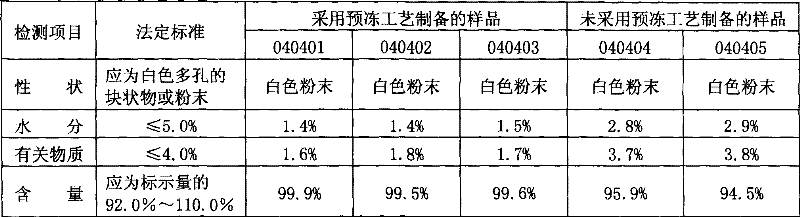
[0029] Take 1.6kg of sodium hydroxide, add appropriate amount of cooling water for injection to prepare sodium hydroxide solution. Take an appropriate amount of water for injection, cool it down, inject sterile nitrogen gas for 15-20 minutes, and then add 12kg of glutathione to make a suspension. Under the condition that both the sodium hydroxide solution and the suspension are fed with sterile nitrogen and airtight, the sodium hydroxide solution is slowly added to the glutathione suspension, and the pH value of the solution is adjusted to 5.2-5.4. Add water for injection to the full amount, continue to pass in sterile nitrogen, stir evenly, and filter aseptically. Fill into shallow freeze-drying trays, fill each tray with 7kg, first keep at -20°C for 3 hours, then rise to -5°C within 2 hours, keep at -5°C for 1 hour, and then drop to - within 1.5 hours Keep it at 42°C for 2 hours, vacuumize, raise the temperature to 50-53°C, keep it warm for 13-14 hours, after freeze-drying ...
Embodiment 2
[0031] Take 1.6kg of sodium hydroxide, add appropriate amount of cooling water for injection to prepare sodium hydroxide solution. Take an appropriate amount of water for injection, cool it down, inject sterile nitrogen gas for 15-20 minutes, and then add 12kg of glutathione to make a suspension. Under the condition that both the sodium hydroxide solution and the suspension are fed with sterile nitrogen and airtight, the sodium hydroxide solution is slowly added to the glutathione suspension, and the pH value of the solution is adjusted to 5.2-5.4. Add water for injection to the full amount, continue to pass in sterile nitrogen, stir evenly, and filter aseptically. Fill into shallow freeze-dried trays, fill each tray with 7kg, first keep at -30°C for 3 hours, then rise to -5°C within 2 hours, keep at -5°C for 1 hour, and then drop to - within 1.5 hours Keep it at 42°C for 2 hours, vacuumize, raise the temperature to 50-53°C, keep it warm for 13-14 hours, after freeze-drying i...
Embodiment 3
[0033] Take 1.6kg of sodium hydroxide, add appropriate amount of cooling water for injection to prepare sodium hydroxide solution. Take an appropriate amount of water for injection, cool it down, inject sterile nitrogen gas for 15-20 minutes, and then add 12kg of glutathione to make a suspension. Under the condition that both the sodium hydroxide solution and the suspension are fed with sterile nitrogen and airtight, the sodium hydroxide solution is slowly added to the glutathione suspension, and the pH value of the solution is adjusted to 5.2-5.4. Add water for injection to the full amount, continue to pass in sterile nitrogen, stir evenly, and filter aseptically. Fill into shallow freeze-drying trays, 7kg per tray, first keep at -20°C for 3 hours, then rise to -1°C within 2 hours, keep at -1°C for 1 hour, and then drop to - within 1.5 hours Keep it at 42°C for 2 hours, vacuumize, raise the temperature to 50-53°C, keep it warm for 13-14 hours, after freeze-drying is complete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com