Method of examining alzheimer s disease and diagnostic reagent
A technology for Alzheimer's disease and testing methods, which can be used in disease diagnosis, material inspection products, biological testing, etc., and can solve problems such as a certain understanding of the amount of Aβ1-42
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1 and 2
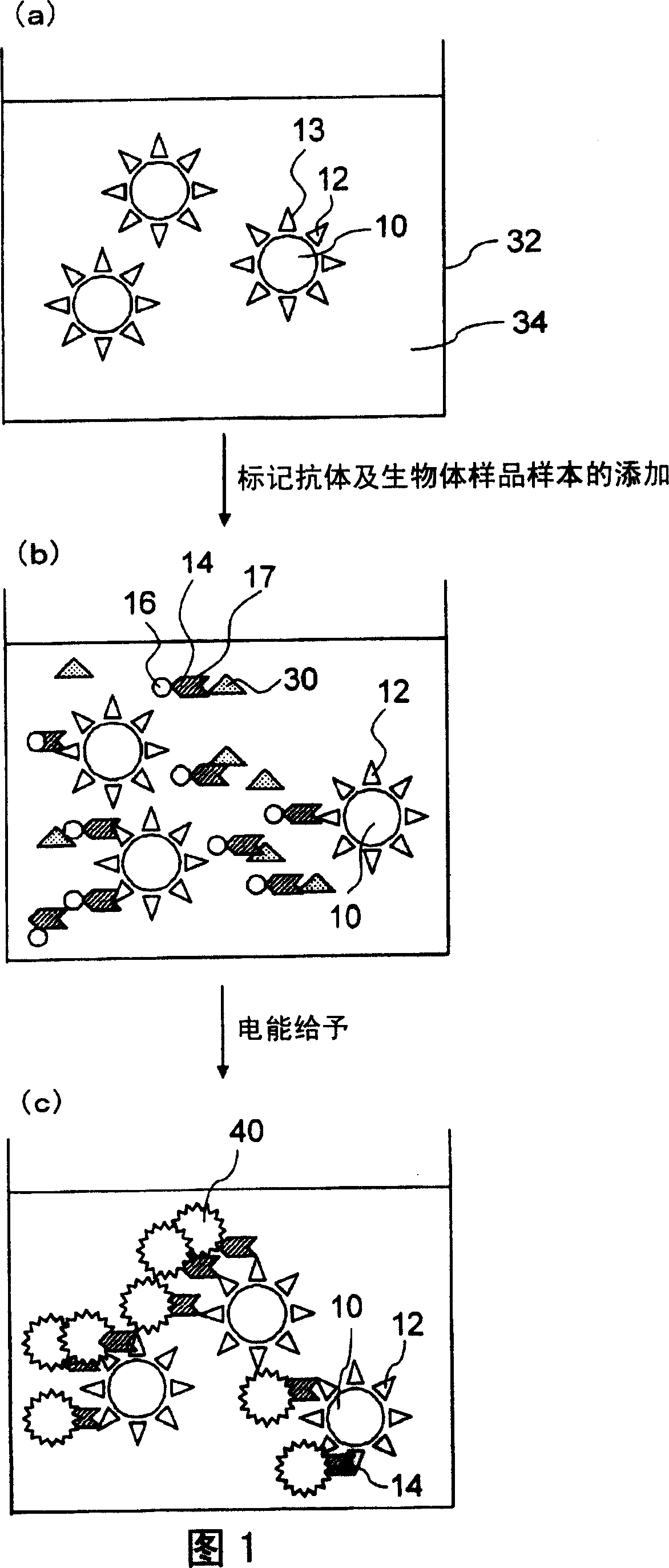
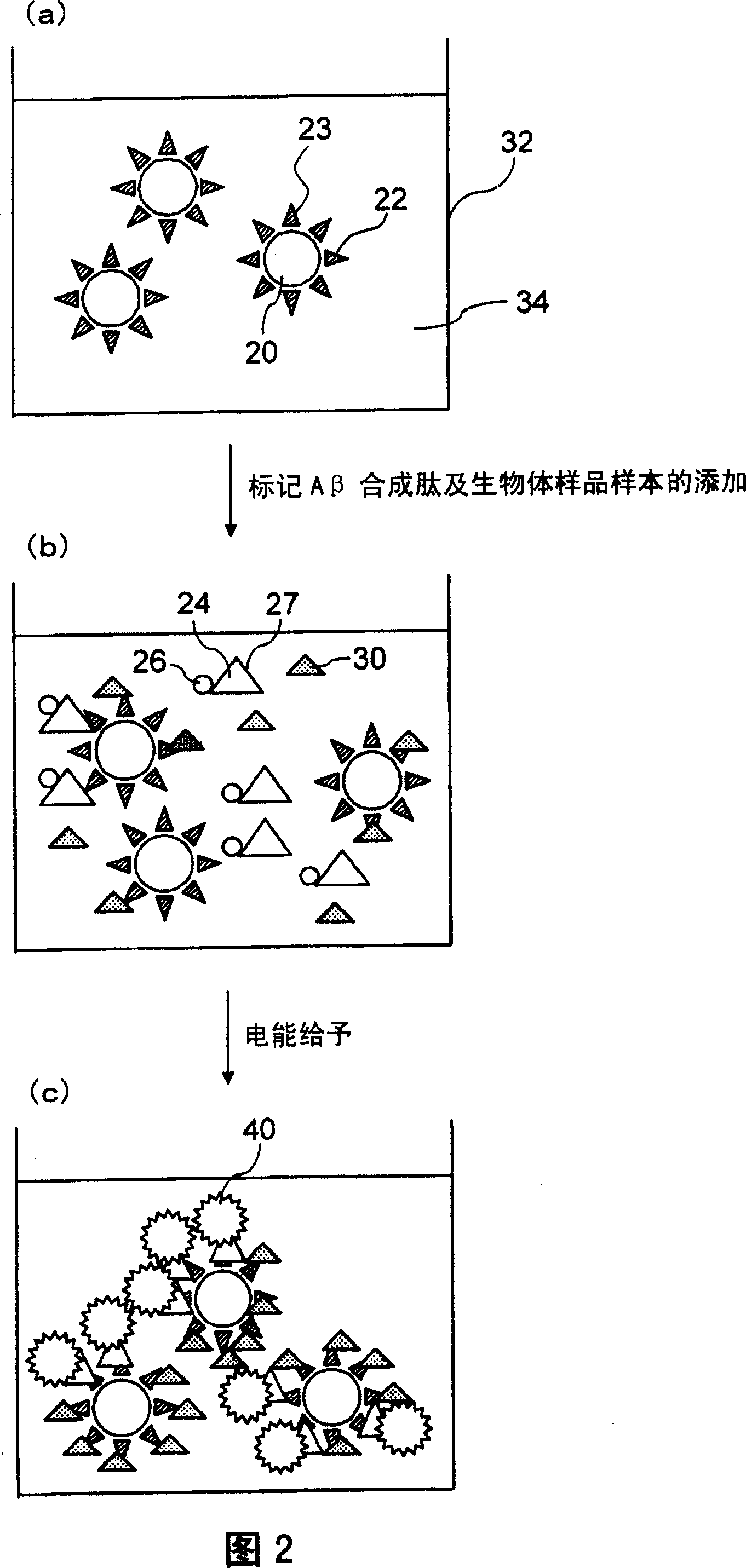
[0119] (Experimental example 1 and 2) Electrochemiluminescence double antibody sandwich assay
[0120] Experimental examples 1 and 2 are analyzes by the electrochemiluminescence double-antibody sandwich assay using two kinds of Aβ-specific antibodies. As the primary antibody, the 21F12 mouse monoclonal antibody (manufactured by Innogenetics), which was prepared by immunizing mice with the 33-42 amino acid site of Aβ1-42, was used as an Aβ1-42 specific reactive antibody. As the secondary antibody, ruthenium-coordinated A 3D6 mouse monoclonal antibody (manufactured by Innogenetics) prepared by immunizing mice with the 1-5 amino acid site of Aβ1-42 was labeled with the compound and used.
[0121] The preparation method of each constituent component of the reagent is described below.
[0122] (1) Preparation method of 21F12 antibody-bound magnetic beads
[0123] Dilute 21F12 mouse monoclonal antibody to 1 mg / mL antibody concentration with 10 mmol / L potassium phosphate buffer (pH...
experiment example 1
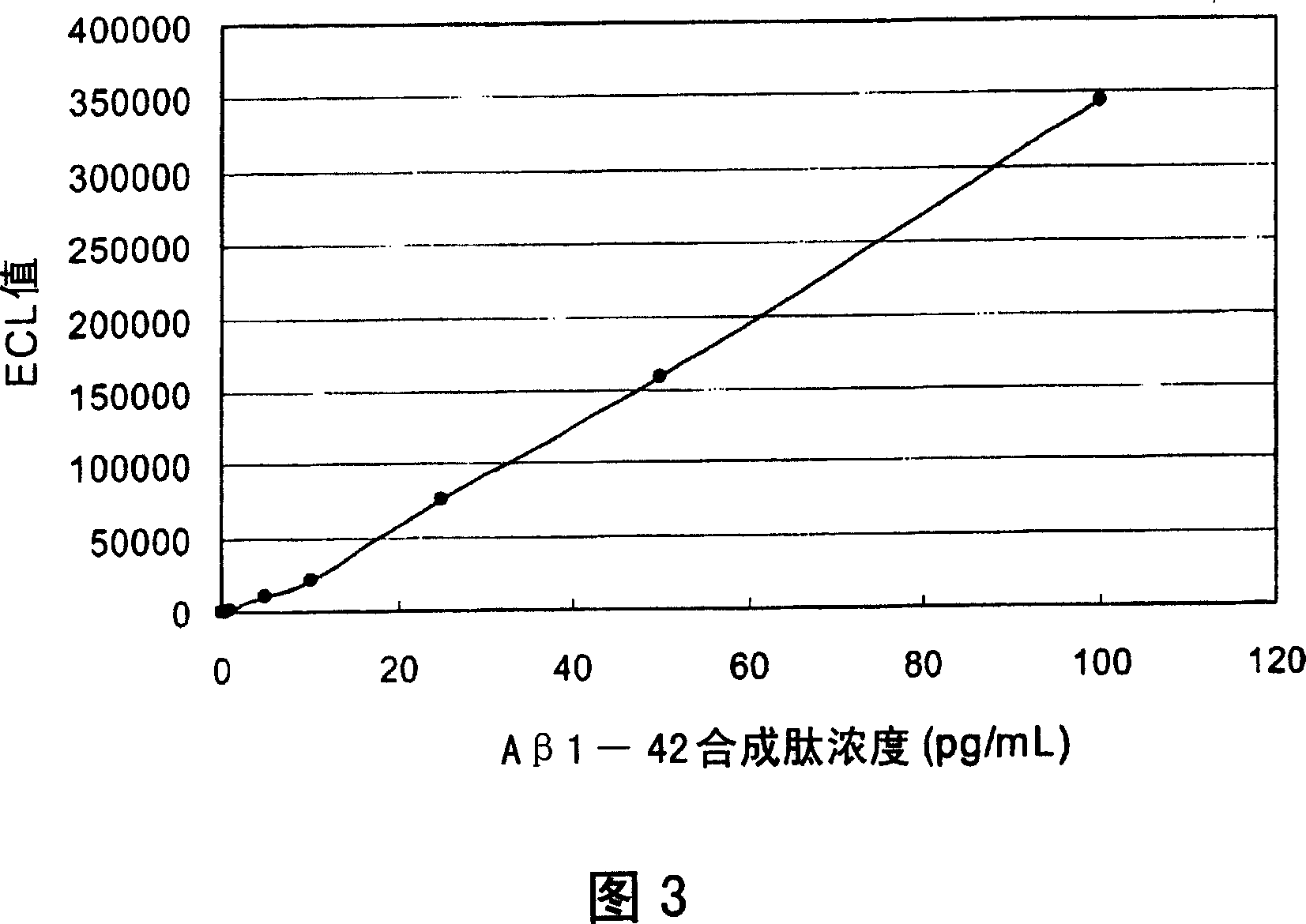
[0127] (Experimental example 1) Determination of Aβ1-42 synthetic peptide
[0128] Add 150 μL of reaction solution [50mmol / L Tris HCl, 1% BSA, 0.15mol / L NaCl, 0.01% (W / V) Tween20, 10mmol / L to a 500 μL polystyrene cup (hereinafter referred to as reaction cup) EDTA2Na, 0.1% normal mouse serum, pH 7.5] (hereinafter referred to as the sandwich measurement reaction solution), prepare 7 parts, and mix and dilute the Aβ1-42 synthetic peptide with the sandwich measurement reaction solution in each cuvette 50 μL of each sample at 0, 0.5, 1, 5, 10, 25, 50 or 100 pg / mL. To this was added 25 μL each of 21F12 antibody-bound magnetic beads diluted to a concentration of 2 mg / mL with a sandwich measurement reaction solution, and reacted at 30° C. for 9 minutes (first reaction).
[0129] Then, after trapping the magnetic beads with a magnet, remove the liquid in the cuvette, and wash with 350 μL of cleaning solution [50 mmol / L Tris HCL, 0.01% (W / V) tween20, 0.15 mol / L NaCl, pH7.5] Magnetic b...
experiment example 2
[0137] (Experimental Example 2) Determination of AD patient and healthy human serum by electrochemiluminescence double antibody sandwich assay
[0138] Add 150 μL of the solution for the sandwich measurement reaction to the cuvette, prepare the necessary amount, mix the solution for the sandwich measurement reaction in each cuvette, and dilute the Aβ1-42 synthetic peptide to 0, 0.5, 1, 5, 10, 25, 50 μL each of samples obtained at 50 or 100 pg / mL (standard for calibration curve preparation), 25 AD patient serum samples, and 25 healthy human serum samples. To this was added 25 μL each of 21F12 antibody-bound magnetic beads diluted to a concentration of 2 mg / mL with a sandwich measurement reaction solution, and reacted at 30° C. for 9 minutes (first reaction).
[0139] Then, after capturing the magnetic beads with a magnet, remove the liquid in the cuvette, and wash the magnetic beads with 350 μL of washing solution [50 mmol / L Tris HCL, 0.01% (W / V) tween20, 0.15 mol / L NaCl, pH7.5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap