Composition and method for removing heavy metals from contaminated samples with membranes comprising purified metallothionein
A technology for metallothionein and heavy metals, applied in the direction of metallothionein, chemical instruments and methods, botany equipment and methods, etc., can solve the problems of poorly understood function or biological purpose, and achieve economical and safe removal and recovery, The effect of high binding affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] In accordance with the teachings of the present invention, the following exemplary protocols describe methods for producing, purifying and analyzing the metal binding proteins of the present invention.
[0108] Sample Preparation
[0109] As a preliminary step to isolate metal-binding proteins, Artemia brine shrimp were cultured in artificial seawater (422.7mM NaCl, 7.24mM KCL, 22.58mM MgCl 2 ·6H 2 O, 25.52mM MgSO 4 ·7H 2 O, 1.33mM CaCl 2 2H 2 O and 0.476 mM NaHCO 3 )middle. Artemia cysts (2.5 g) were cultured for 48 hours in 250 ml of AS supplemented with antibiotics at 30°C and rotation at 125 rpm. After 24 hours, phototropic Artemia was harvested, incubated for an additional 24 hours, and collected by filtration through filter cloth. Shrimp were weighed and stored at -80°C if not used immediately.
[0110] Artemia was then homogenized in homogenization buffer (HB) (10 mM Tris-HCl (pH 8.0), 0.1 mM DTT, 0.5 mM PMSF, and 10 μg / ml soybean trypsin inhibitor) and ...
Embodiment 2
[0116] Example 2 Cloning and sequencing of the gene encoding Artemia metallojunction protein
[0117] Total RNA was extracted from 48-hour nauplius (larval stage of Artemia) using the RNAzol method. A 48 hour sample of nauplii was prepared as described in Example 1. mRNA was then isolated from total RNA samples using the PolyTract Procedure (Promega, WI). cDNA was generated from mRNA using Superscript and the 3'RACE kit protocol (cat# 18373, Gibco / BRL, Wl), which was used in the synthesis reactions described below.
[0118] cDNA synthesis reaction:
[0119] Artemia mRNA 25μl (500ng)
[0120] DEPC H 2O 30 μl
[0121] 10 μM AP 5 μl
[0122] Incubate the above mixture at 70 °C for 10 min, then place on ice for 1-2 min. Volatile liquid was collected by centrifugation at 10000 rpm for 10 seconds. Add the following to the above RNA mixture to generate a cDNA solution:
[0123] 10x PCR buffer 10μl
[0124] 25mM MgCl 2 10μl
[0125] 10mM dNTP 5μl
[0126] 0.1mM DTT ...
Embodiment 3
[0159] Example 3 Transgenic tobacco expression of Artemia MT
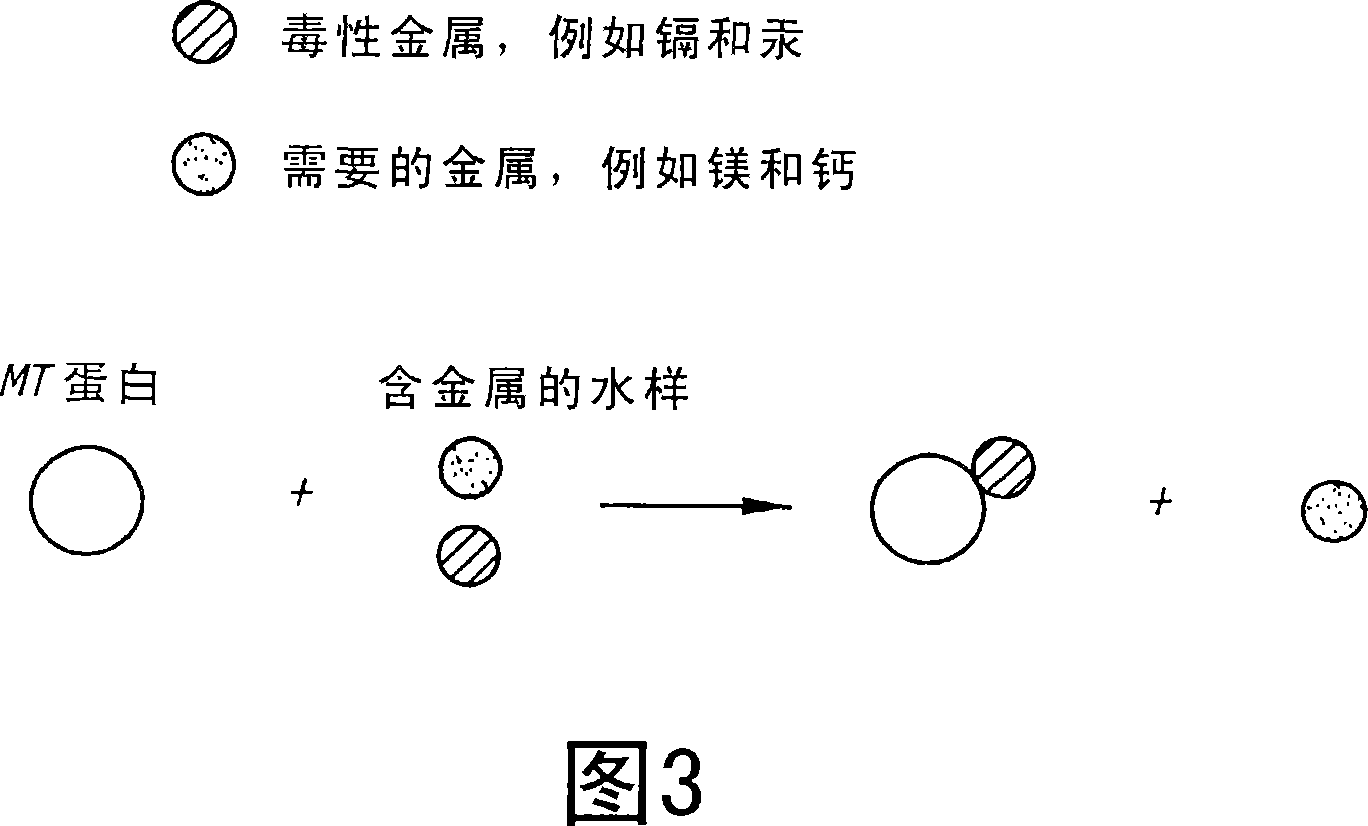
[0160] Exemplary studies are provided below that can be performed on any of the novel metal binding proteins of the invention to assist in the validation of whether the protein is a metal binding protein. For example, metal binding proteins of the invention can bind heavy metals such as zinc, cadmium and copper. The ability of the isolated protein to bind heavy metals is described and elaborated in the transformation of E. coli with exemplary MTs of the invention disclosed herein, which is shown in FIG. 1 .
[0161] As previously described, modified organisms for the production of the novel metal-binding proteins of the invention can be produced following the teachings provided herein. Exemplary modified organisms include transgenic tobacco plants, which are particularly useful in the methods described herein.
[0162] The cDNA of MT used for cloning into the TOPO.CR2 vector is called pART mt . The co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com