Method for preparing oral non-ester-type autibiotic cetprozil
A technology of cefprozil and cephalosporanic acid, applied in the direction of organic chemistry, can solve the problems of harsh reaction conditions, difficult industrial production, and low reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
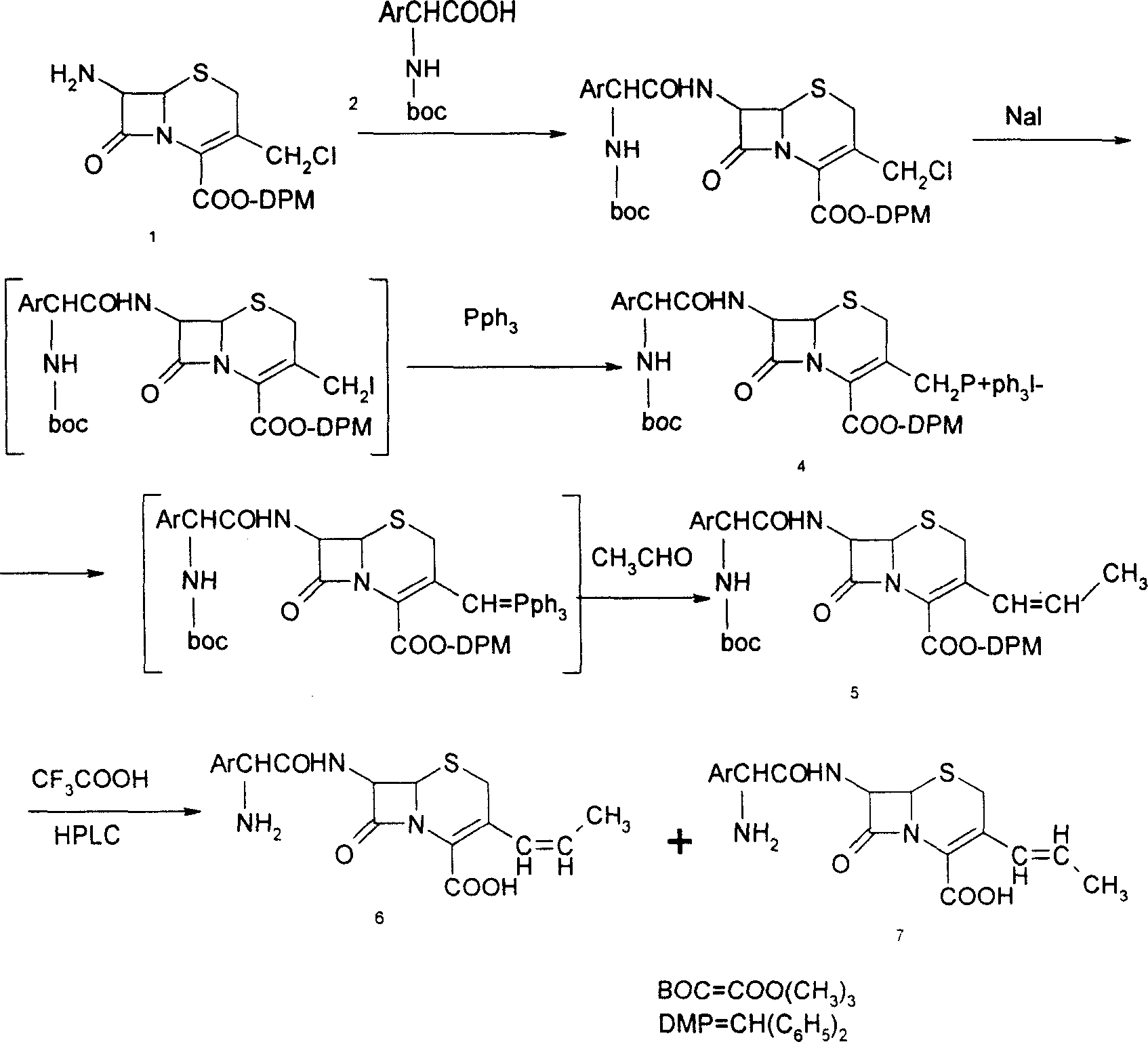
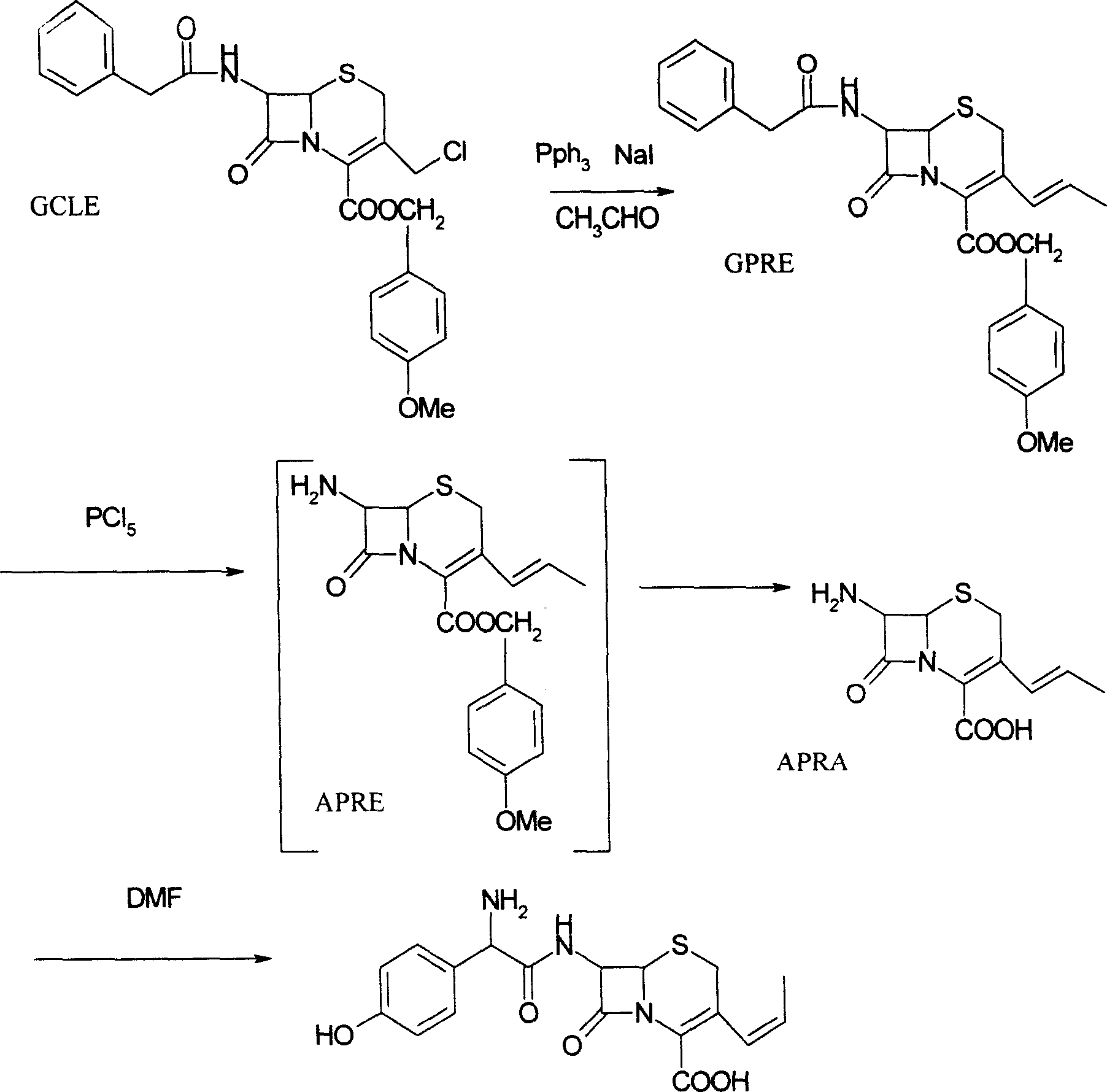
[0020] 1, Preparation of 7-phenylacetamido-3-(prop-1-enyl)-4-cephalosporanic acid p-methoxybenzyl ester (GPRE)
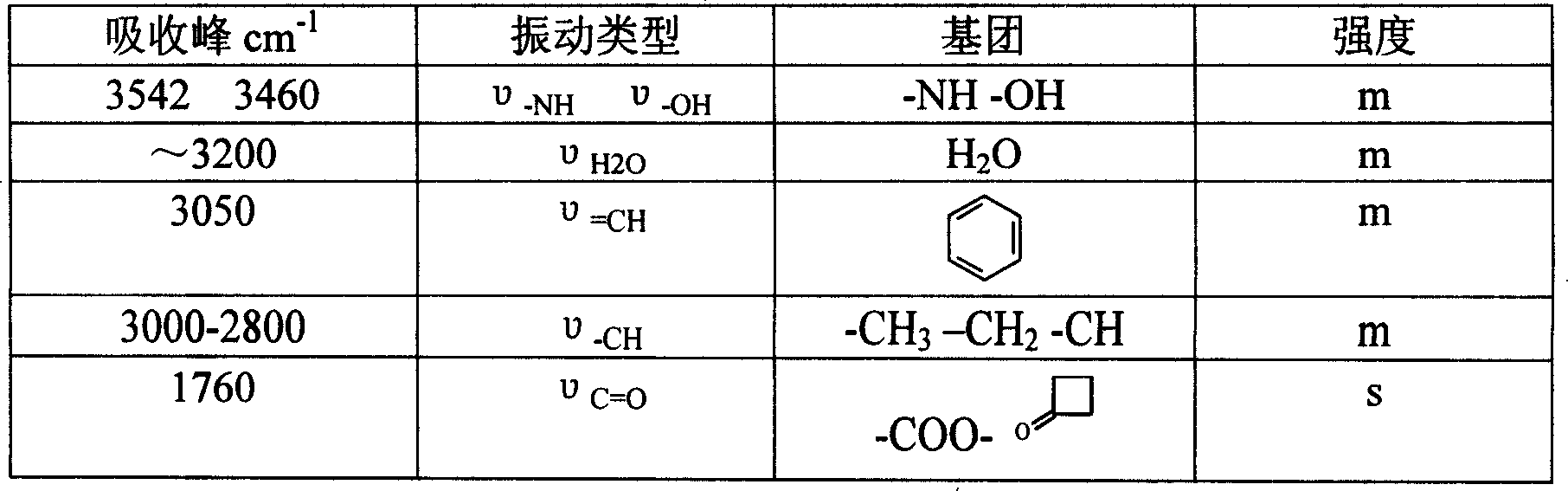
[0021] Put 50g (102.7mmol) of GCLE, 16.2g (107.8mmol) of sodium iodide, and 28.3g (107.8mmol) of triphenylphosphine into a 1L reaction bottle, stir, add 300ml of dichloromethane and 300ml of water, and set the temperature at 26~ React at 28°C for 2 hours. Separate the organic phase, cool to 0°C, add 0.5N sodium hydroxide solution, stir for about 3 hours, collect the organic phase, wash with water (200ml), separate the water layer, add 200ml of dichloromethane, cool to -10°C , 250 ml of 2-propanol and 130 ml of 40% acetaldehyde were added, and stirred for 16 hours. Add 200ml of 20% potassium thiosulfate aqueous solution, stir for 25 minutes, separate the organic phase, then add 200ml of 20% potassium thiosulfate aqueous solution, stir for 10 minutes, separate the organic phase, wash with water (300ml), and collect the organic phase , concentrated by rotary evaporat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com