Method of detecting JAK2V617F mutation and its special primer and TaqMan MGB probe
A JAK2V617F and probe technology is applied in the field of detecting JAK2V617F mutation and its special primers and TaqMan-MGB probes, which can solve the problems of lack of primers and specific fluorescent probes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1. Design of primers and TaqMan-MGB probes for detection of myeloproliferative disease JAK2V617F mutation
[0050] 1. Primers and TaqMan-MGB probes designed for the detection of myeloproliferative disease JAK2V617F mutation based on the genome gene of JAK2
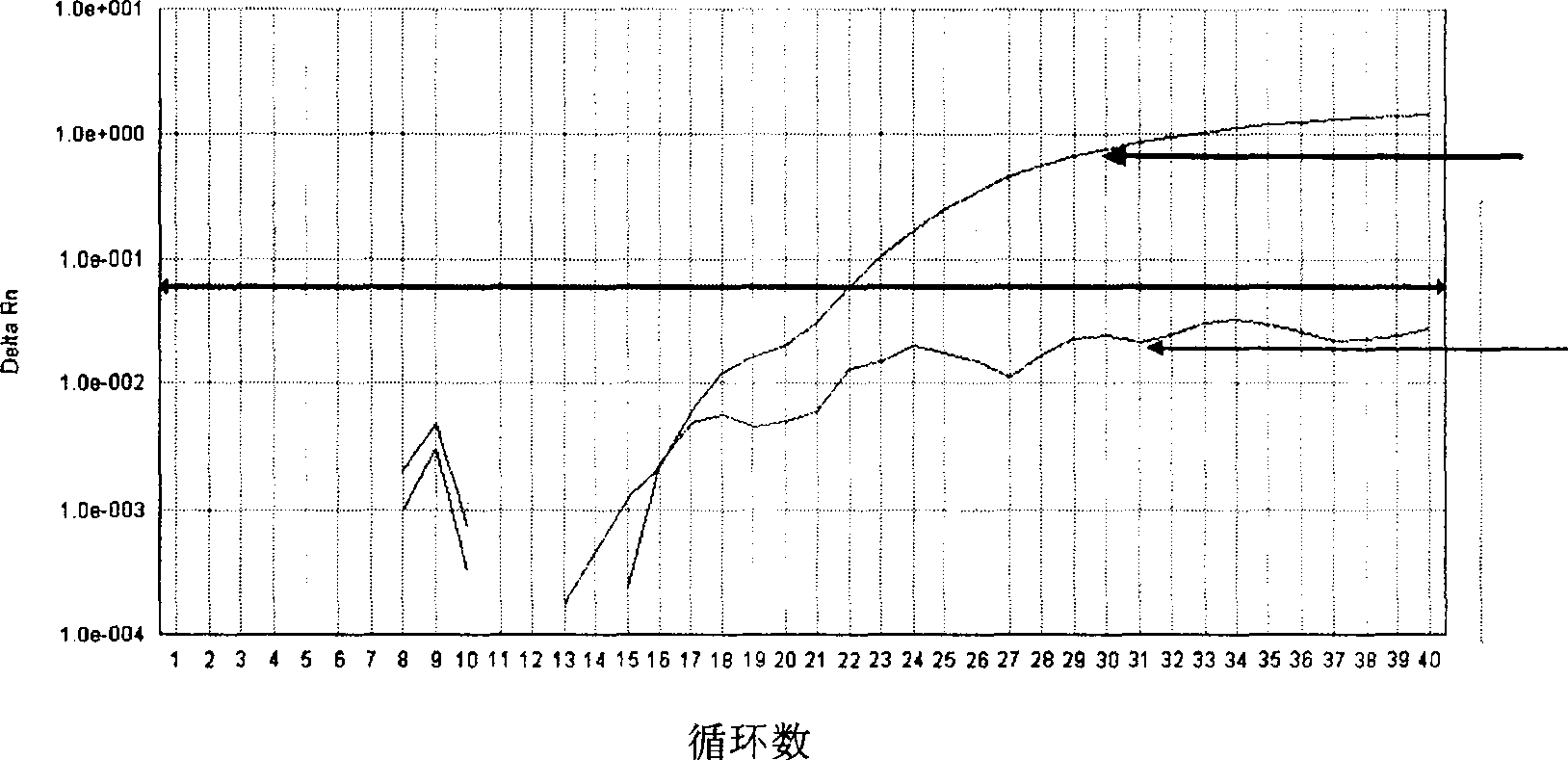
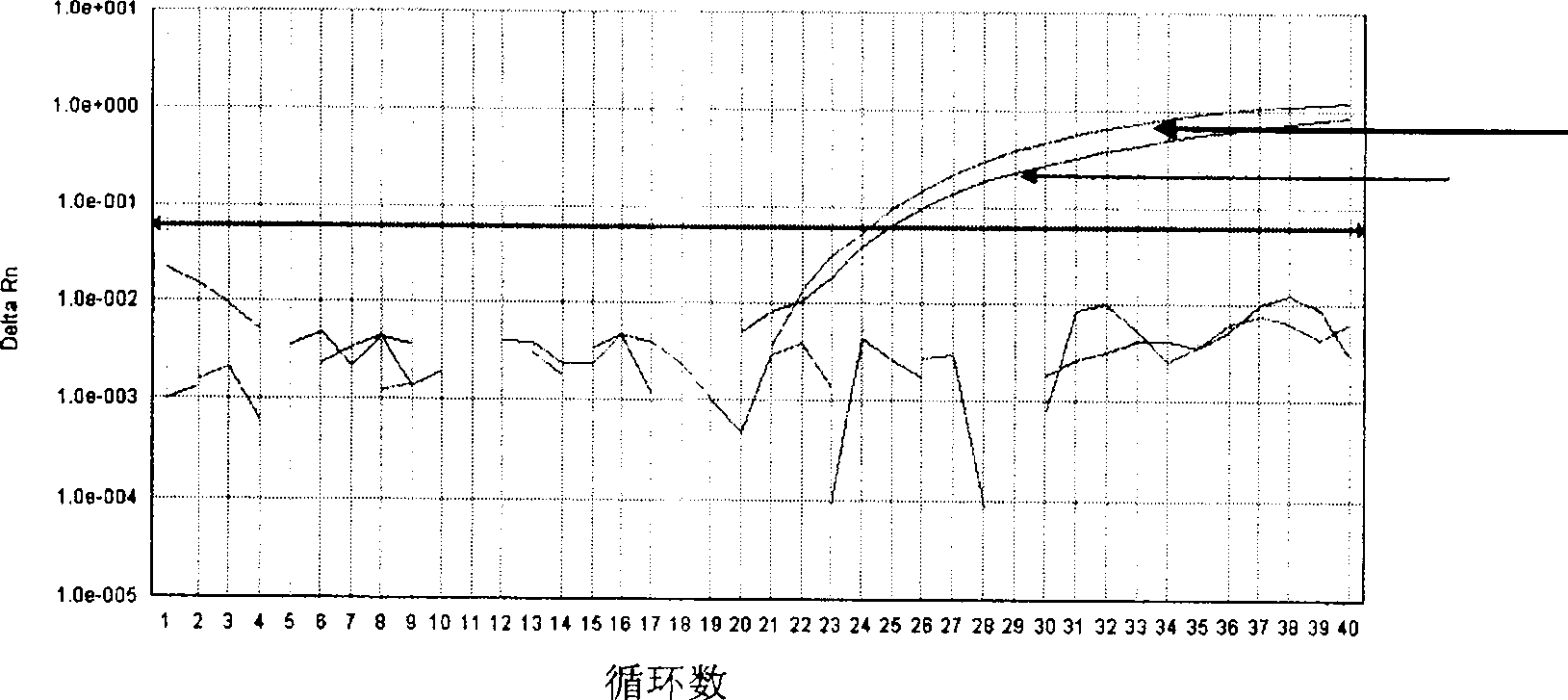
[0051] According to the gene sequence (GenBank number of the wild type JAK2 gene: NT_008413, JAK2V617F mutant gene is the codon GTC of valine at position 617 of the wild-type gene mutated to the codon TTC of phenylalanine) and its reverse complementary sequence is designed with PrimerExpress Software 2.0 software. PCR primers, and two TaqMan-MGB probes were designed according to the reverse complementary sequences of the wild-type JAK2 kinase gene and the JAK2 kinase JAK2V617F mutant gene, and the 5' ends of the two TaqMan-MGB probes were respectively labeled with the reporter fluorescent group FAM( Red fluorescence) and VIC (green fluorescence), the 3' end is labeled with a non-luminescent quencher group, ...
Embodiment 2
[0062] Example 2. Detection of JAK2V617F mutation in myeloproliferative disease
[0063] 1. Detection of JAK2V617F mutation in myeloproliferative diseases using primers designed according to the JAK2 genome gene and TaqMan-MGB probe
[0064] Use the primers and TaqMan-MGB probes designed according to the JAK2 genome gene in step one of Example 1 for MPDs patients, including PV, ET, MF, unclassified MPDs, and AML, CML, ALL, CLL and non-Hodgkin Myeloproliferative disorders JAK2V617F For the detection of mutations, the normal donor bone marrow (NDM) was used as a control at the same time. The specific method is as follows:
[0065] 1) Extraction of genomic DNA from the sample to be tested
[0066] Genomic DNA was extracted from bone marrow samples of MPDs patients and normal bone marrow transplantation donors (see Table 3) under aseptic conditions according to the DNA zol kit (purchased from Gibco, USA) and referring to the kit instructions. The method was: anticoagulated with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com