Preparation method of CYP1A2 polypeptide and antihuman CYP1A2 polypeptide antibody
A CYP1A2 and peptide technology, applied in the field of peptides, can solve the problems of high price of human CYP1A2 and high cost of antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0006] Embodiment 1: The amino acid sequence of the CYP1A2 polypeptide in this embodiment is: GlyArgGluArgArgProArgLeuSerAspArgProGlnLeuPro.
specific Embodiment approach 2
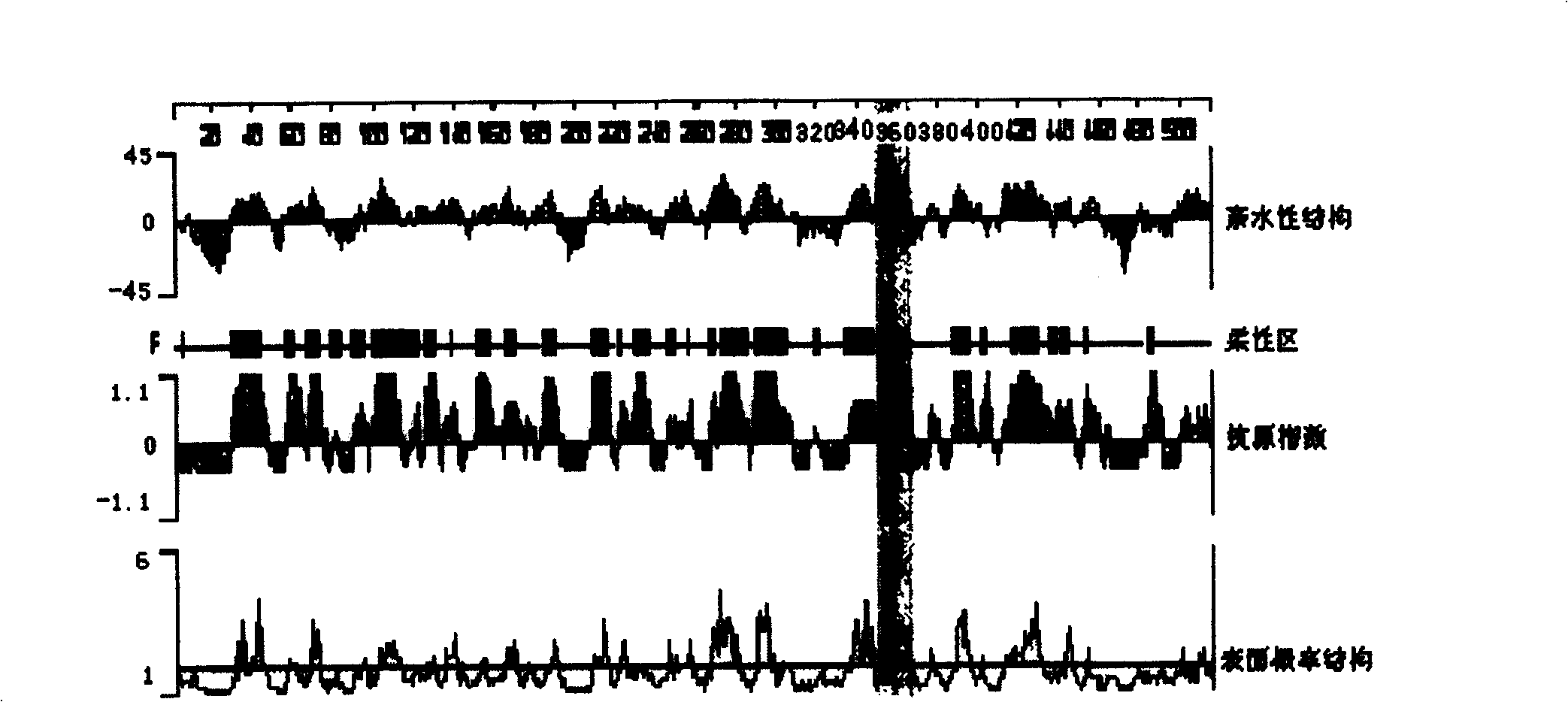
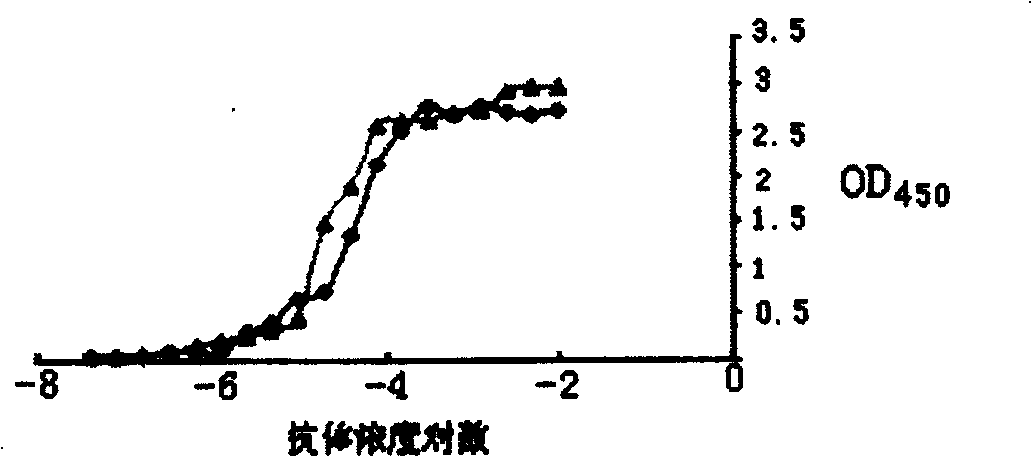
[0007] Specific embodiment two: In this embodiment, the anti-human CYP1A2 polypeptide antibody is prepared according to the following steps: (1) Analysis of CYP1A2 epitope: use DNAStar software analysis to determine the 15 amino acids at positions 351-365 of the CYP1A2 protein as the amino acid sequence of the synthetic polypeptide; ( 2) Synthesis of CYP1A2 polypeptide: Synthesized and purified by an automatic polypeptide synthesizer; (3) Cross-linking of CYP1A2 polypeptide and carrier protein: CYP1A2 polypeptide and carrier protein KLH are linked by glutaraldehyde, and then boric acid buffer with a pH value of 8.5 Dialyze for 8-12 hours, cross-link to form CYP1A2-KLH; (4) Preparation of rabbit anti-CYP1A2 polypeptide antibody: inject the emulsified CYP1A2-KLH subcutaneously on the back of the rabbit, and repeatedly boost the immunization until the antibody titer is equal to or higher than 1: 10000; (5) collecting and separating serum containing rabbit anti-human CYP1A2 polypep...
specific Embodiment approach 3
[0009] Specific embodiment three: the difference between this embodiment and specific embodiment two is: in step (two), a conventional C18 RP-HPLC column is used for purification. Other steps are the same as the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com