N-terminal polypeptide of retinoic acid induced protein 16 and preparation method and application of antibody thereof
A technology for inducing protein and polypeptide antibodies, applied in the field of biomedicine, can solve the problems that antibodies cannot satisfy chemical experiments and immunofluorescence experiments at the same time, RAI16 is expensive, and the cost of antibodies is high, and achieves good specificity, high titer, and low preparation cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Now, the present invention will be further described in conjunction with the embodiments, but the implementation of the present invention is not limited thereto. Example 1: Preparation of anti-RAI16 N-terminal polypeptide antibody
[0031] The anti-RAI16 N-terminal polypeptide antibody was prepared according to the following steps:
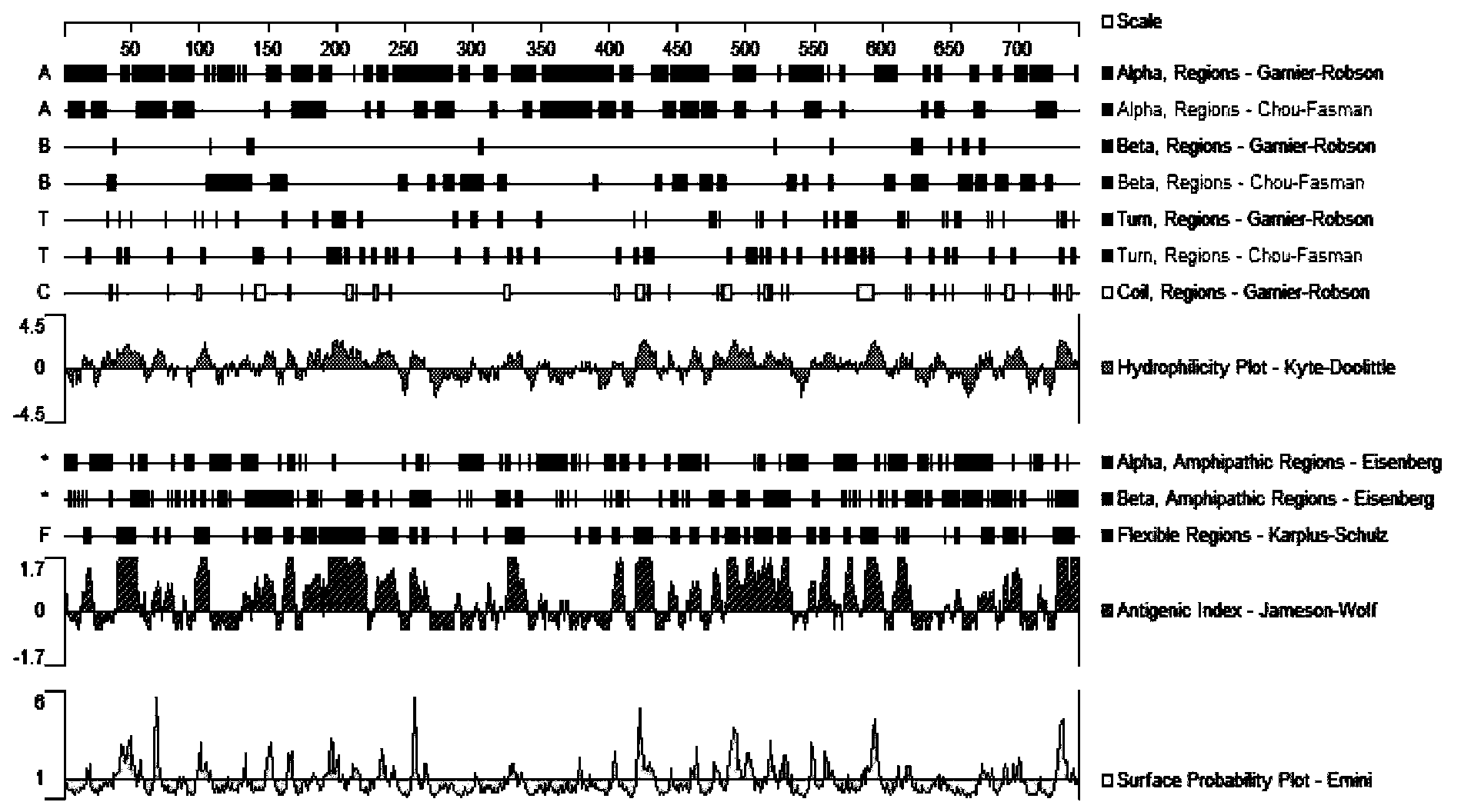
[0032] Analysis of the RAI16 N-terminal epitope: DNAStar software was used to analyze the amino acid sequence of the N-terminal of the RAI16 protein, and the analysis results were as follows figure 1 As shown, the 40-55 amino acids (polypeptide) at the N-terminal of the RAI16 protein have excellent hydrophilic structure, flexible region, antigenic index and surface probability structure. Determine the 16 amino acids at positions 40-55 of the RAI16 protein as the amino acid sequence of the synthetic polypeptide:
[0033] Cys-Thr-Asp-Glu-Ser-Thr-Pro-Ala-Lys-Lys-Thr-Asp-Ile-Pro-Trp-Arg
[0034] Synthesis of RAI16 polypeptide: synthesized by...
Embodiment 2
[0043] Example 2: Determination of the Titer of Anti-RAI16 N-terminal Polypeptide Antibody
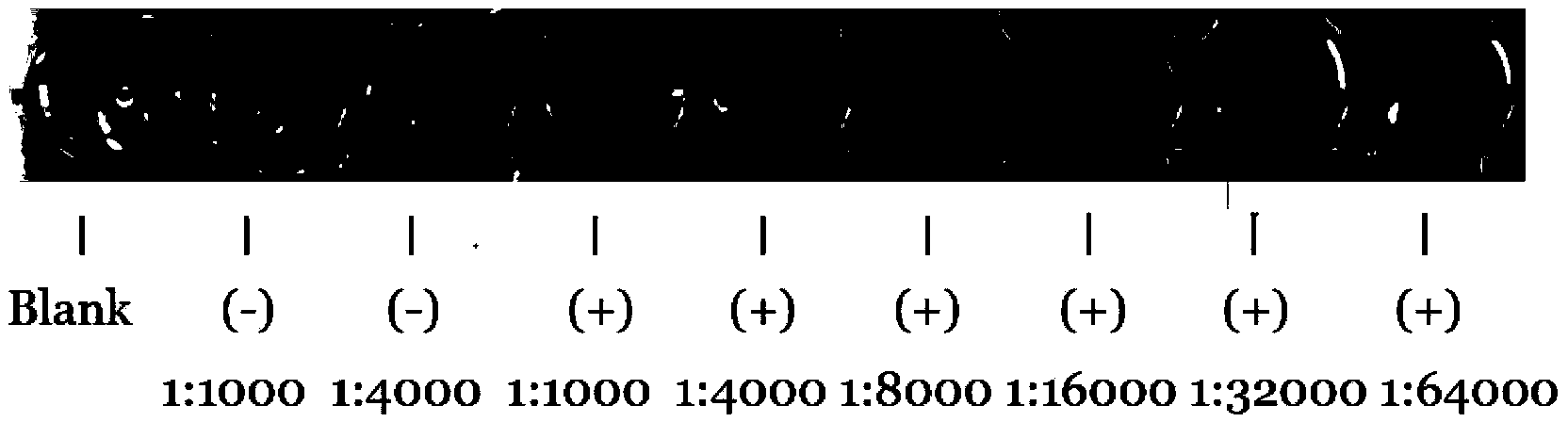
[0044] In this embodiment, ELISA is used to detect the titer of the anti-RAI16 N-terminal polypeptide antibody: the ELISA detection plate is coated with RAI16-BSA at a concentration of 1mg / L, 100ul per well, incubated at 4°C for 8-12h, and then 200ul is added to each well Bovine serum with a concentration of 100ul was blocked at 37°C for 2h, and after washing, add double-diluted anti-RAI16 polypeptide antibody (normal rabbit serum was added to the control group), incubate at 37°C for 60min, and add to each well after washing three times. 1:5000 diluted HRP-labeled goat anti-rabbit IgG100ul, incubated at 37°C for 30min, washed 3 times, then developed TMB color, measured the absorbance value of the sample at A450 with a microplate reader, and calculated the antibody titer. The potency is 1:64000 (eg image 3 shown).
Embodiment 3
[0045] Example 3: Anti-RAI16 N-terminal polypeptide antibody is used for detection of RAI16 protein expression
[0046] 1. Western blotting to detect the expression of RAI16 in human liver cancer cell line HepG2 cells and hepatocellular carcinoma tissue samples
[0047] HepG2 cell and hepatocellular carcinoma tissue protein sample preparation: 1×10 7 HepG2 cells or 100mg hepatocellular carcinoma tissue (HCC, after homogenization), add lysate (50mM Tris-HCl, 150mM NaCl, 0.5mM EDTA, protease inhibitor cocktail) and lyse at 4°C for 30min, under the conditions of 4°C and 10000g After centrifugation for 20 min, the protein in the supernatant was quantified, aliquoted and stored at -80°C. In this embodiment, the immunoblotting analysis of the antibody is carried out, and the specificity of the antibody is identified by a synthetic polypeptide competition method. Suspend the prepared human HepG2 cells or hepatocellular carcinoma tissue in 5xSDS loading buffer, heat at 95°C for 10 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com