How to Test Rhodochrosite's Corrosion Resistance
OCT 1, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Rhodochrosite Corrosion Testing Background and Objectives
Rhodochrosite, a manganese carbonate mineral (MnCO₃), has gained increasing attention in various industrial applications due to its unique properties. The study of its corrosion resistance has evolved significantly over the past decades, transitioning from basic qualitative assessments to sophisticated quantitative analyses. This evolution reflects the growing importance of understanding how this mineral responds to different corrosive environments, particularly as its applications expand beyond decorative uses to more functional roles in industrial settings.
The historical context of rhodochrosite corrosion testing dates back to the mid-20th century when mineralogists began systematic studies of carbonate mineral degradation. Early research primarily focused on visual inspection and weight loss measurements, providing only rudimentary insights into corrosion mechanisms. The 1980s marked a turning point with the introduction of electrochemical testing methods, enabling more precise characterization of rhodochrosite's behavior in various solutions.
Recent technological advancements have revolutionized testing methodologies, incorporating advanced surface analysis techniques such as X-ray photoelectron spectroscopy (XPS), atomic force microscopy (AFM), and scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDS). These developments have significantly enhanced our ability to understand the microscopic changes occurring during corrosion processes.
The primary objective of rhodochrosite corrosion testing is to establish standardized protocols for evaluating its resistance across diverse environmental conditions. This includes determining corrosion rates in various pH levels, temperatures, and chemical exposures relevant to industrial applications. Additionally, there is a critical need to understand the influence of mineral composition variations, as natural rhodochrosite samples can contain different impurities that potentially affect corrosion behavior.
Another key goal is to develop predictive models that can accurately forecast rhodochrosite's long-term performance in specific environments. Such models would enable more informed material selection decisions for applications where corrosion resistance is paramount. This becomes particularly important as rhodochrosite finds new applications in specialized fields such as environmental remediation and certain electronic components.
The technical trajectory suggests a growing emphasis on sustainable testing methods that minimize environmental impact while maximizing data reliability. This includes the development of non-destructive testing techniques and accelerated testing protocols that can provide meaningful results without requiring extensive time periods or sample destruction.
Understanding rhodochrosite's corrosion mechanisms also serves broader scientific objectives, contributing to fundamental knowledge about manganese carbonate chemistry and potentially informing the development of synthetic materials with enhanced corrosion resistance properties inspired by this natural mineral's behavior.
The historical context of rhodochrosite corrosion testing dates back to the mid-20th century when mineralogists began systematic studies of carbonate mineral degradation. Early research primarily focused on visual inspection and weight loss measurements, providing only rudimentary insights into corrosion mechanisms. The 1980s marked a turning point with the introduction of electrochemical testing methods, enabling more precise characterization of rhodochrosite's behavior in various solutions.
Recent technological advancements have revolutionized testing methodologies, incorporating advanced surface analysis techniques such as X-ray photoelectron spectroscopy (XPS), atomic force microscopy (AFM), and scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDS). These developments have significantly enhanced our ability to understand the microscopic changes occurring during corrosion processes.
The primary objective of rhodochrosite corrosion testing is to establish standardized protocols for evaluating its resistance across diverse environmental conditions. This includes determining corrosion rates in various pH levels, temperatures, and chemical exposures relevant to industrial applications. Additionally, there is a critical need to understand the influence of mineral composition variations, as natural rhodochrosite samples can contain different impurities that potentially affect corrosion behavior.
Another key goal is to develop predictive models that can accurately forecast rhodochrosite's long-term performance in specific environments. Such models would enable more informed material selection decisions for applications where corrosion resistance is paramount. This becomes particularly important as rhodochrosite finds new applications in specialized fields such as environmental remediation and certain electronic components.
The technical trajectory suggests a growing emphasis on sustainable testing methods that minimize environmental impact while maximizing data reliability. This includes the development of non-destructive testing techniques and accelerated testing protocols that can provide meaningful results without requiring extensive time periods or sample destruction.
Understanding rhodochrosite's corrosion mechanisms also serves broader scientific objectives, contributing to fundamental knowledge about manganese carbonate chemistry and potentially informing the development of synthetic materials with enhanced corrosion resistance properties inspired by this natural mineral's behavior.
Market Applications and Demand Analysis for Corrosion-Resistant Materials
The global market for corrosion-resistant materials has experienced significant growth in recent years, driven primarily by increasing industrial activities in developing economies and the rising need for durable infrastructure. The current market value for corrosion-resistant materials exceeds $25 billion, with a compound annual growth rate projected at 5.7% through 2028, according to recent industry analyses.
Rhodochrosite, a manganese carbonate mineral, represents an emerging segment within this market. While traditionally valued for ornamental purposes, its potential corrosion-resistant properties have attracted attention from various industrial sectors. The demand for alternative corrosion-resistant materials has intensified as industries seek cost-effective solutions that can withstand increasingly harsh operating environments.
The oil and gas industry constitutes a major market for corrosion-resistant materials, particularly for offshore installations where seawater exposure accelerates corrosion processes. Equipment manufacturers report that corrosion-related maintenance accounts for approximately 15% of operational costs in offshore operations, creating substantial demand for innovative solutions like rhodochrosite-based coatings or composites.
Chemical processing industries represent another significant market segment, where exposure to acids, bases, and other corrosive substances necessitates specialized materials. The pharmaceutical sector, with its stringent requirements for material purity and chemical stability, has shown particular interest in natural mineral-based solutions that offer both corrosion resistance and biocompatibility.
Infrastructure development, especially in coastal regions and areas with extreme weather conditions, drives demand for corrosion-resistant construction materials. The rehabilitation of aging infrastructure in developed nations further amplifies this demand, with government spending on infrastructure renewal projects reaching record levels in North America and Europe.
Marine applications present a specialized but lucrative market for corrosion-resistant materials. Ship builders, offshore wind farm developers, and underwater cable manufacturers all require materials capable of withstanding prolonged seawater exposure. The expanding blue economy initiatives worldwide have created new opportunities for innovative materials in this sector.
Electronics manufacturing represents an emerging application area, where miniaturization trends have increased sensitivity to even microscopic corrosion damage. The semiconductor industry's ultra-pure manufacturing environments demand materials with exceptional corrosion resistance and minimal particle generation.
Market research indicates that customers across these sectors prioritize long-term performance over initial cost, with lifecycle cost analysis increasingly driving purchasing decisions. This trend favors materials like rhodochrosite that may offer superior durability despite potentially higher upfront costs compared to conventional options.
Rhodochrosite, a manganese carbonate mineral, represents an emerging segment within this market. While traditionally valued for ornamental purposes, its potential corrosion-resistant properties have attracted attention from various industrial sectors. The demand for alternative corrosion-resistant materials has intensified as industries seek cost-effective solutions that can withstand increasingly harsh operating environments.
The oil and gas industry constitutes a major market for corrosion-resistant materials, particularly for offshore installations where seawater exposure accelerates corrosion processes. Equipment manufacturers report that corrosion-related maintenance accounts for approximately 15% of operational costs in offshore operations, creating substantial demand for innovative solutions like rhodochrosite-based coatings or composites.
Chemical processing industries represent another significant market segment, where exposure to acids, bases, and other corrosive substances necessitates specialized materials. The pharmaceutical sector, with its stringent requirements for material purity and chemical stability, has shown particular interest in natural mineral-based solutions that offer both corrosion resistance and biocompatibility.
Infrastructure development, especially in coastal regions and areas with extreme weather conditions, drives demand for corrosion-resistant construction materials. The rehabilitation of aging infrastructure in developed nations further amplifies this demand, with government spending on infrastructure renewal projects reaching record levels in North America and Europe.
Marine applications present a specialized but lucrative market for corrosion-resistant materials. Ship builders, offshore wind farm developers, and underwater cable manufacturers all require materials capable of withstanding prolonged seawater exposure. The expanding blue economy initiatives worldwide have created new opportunities for innovative materials in this sector.
Electronics manufacturing represents an emerging application area, where miniaturization trends have increased sensitivity to even microscopic corrosion damage. The semiconductor industry's ultra-pure manufacturing environments demand materials with exceptional corrosion resistance and minimal particle generation.
Market research indicates that customers across these sectors prioritize long-term performance over initial cost, with lifecycle cost analysis increasingly driving purchasing decisions. This trend favors materials like rhodochrosite that may offer superior durability despite potentially higher upfront costs compared to conventional options.
Current Challenges in Rhodochrosite Corrosion Testing
Testing the corrosion resistance of rhodochrosite presents several significant challenges that researchers and industry professionals must overcome. The mineral's complex composition, primarily manganese carbonate (MnCO₃), exhibits variable behavior under different environmental conditions, making standardized testing protocols difficult to establish. This variability stems from natural impurities and structural differences in samples from different geological sources.
One major challenge is the lack of universally accepted testing methodologies specifically designed for rhodochrosite. While ASTM and ISO standards exist for corrosion testing of metals and some minerals, rhodochrosite's unique properties require specialized approaches. Researchers often must adapt existing protocols, leading to inconsistencies in results across different studies and laboratories.
Environmental simulation presents another significant hurdle. Rhodochrosite applications span diverse environments—from acidic industrial settings to marine exposures—each requiring different test parameters. Creating accelerated testing conditions that accurately predict long-term performance without introducing artificial degradation mechanisms remains problematic. The correlation between accelerated laboratory tests and real-world performance continues to be questionable.
Sample preparation introduces additional complications. Rhodochrosite's natural heterogeneity means that test specimens may not represent the bulk material accurately. Surface preparation techniques can significantly alter corrosion behavior, with polishing, grinding, or other finishing methods potentially creating misleading results. The mineral's relatively soft nature (3.5-4 on Mohs scale) makes consistent surface preparation particularly challenging.
Analytical measurement techniques also present limitations. Traditional weight loss methods lack sensitivity for slow corrosion processes, while electrochemical techniques may be complicated by rhodochrosite's semiconducting properties. Advanced surface analysis techniques like XPS and ToF-SIMS require expensive equipment and specialized expertise not readily available in many testing facilities.
Data interpretation remains problematic due to the complex corrosion mechanisms involved. Rhodochrosite can undergo multiple simultaneous degradation processes, including dissolution, oxidation, and transformation to other manganese compounds. Distinguishing between these mechanisms and quantifying their relative contributions requires sophisticated analytical approaches.
Reproducibility issues further complicate testing efforts. Even with carefully controlled protocols, test-to-test variability can be substantial due to subtle differences in sample composition, microstructure, and environmental conditions. This variability undermines confidence in test results and makes comparative evaluations difficult.
Finally, there is a significant knowledge gap regarding the long-term corrosion behavior of rhodochrosite. Most existing studies focus on short-term exposure, leaving uncertainty about performance over decades of service life. Developing reliable predictive models based on accelerated testing remains an unsolved challenge in the field.
One major challenge is the lack of universally accepted testing methodologies specifically designed for rhodochrosite. While ASTM and ISO standards exist for corrosion testing of metals and some minerals, rhodochrosite's unique properties require specialized approaches. Researchers often must adapt existing protocols, leading to inconsistencies in results across different studies and laboratories.
Environmental simulation presents another significant hurdle. Rhodochrosite applications span diverse environments—from acidic industrial settings to marine exposures—each requiring different test parameters. Creating accelerated testing conditions that accurately predict long-term performance without introducing artificial degradation mechanisms remains problematic. The correlation between accelerated laboratory tests and real-world performance continues to be questionable.
Sample preparation introduces additional complications. Rhodochrosite's natural heterogeneity means that test specimens may not represent the bulk material accurately. Surface preparation techniques can significantly alter corrosion behavior, with polishing, grinding, or other finishing methods potentially creating misleading results. The mineral's relatively soft nature (3.5-4 on Mohs scale) makes consistent surface preparation particularly challenging.
Analytical measurement techniques also present limitations. Traditional weight loss methods lack sensitivity for slow corrosion processes, while electrochemical techniques may be complicated by rhodochrosite's semiconducting properties. Advanced surface analysis techniques like XPS and ToF-SIMS require expensive equipment and specialized expertise not readily available in many testing facilities.
Data interpretation remains problematic due to the complex corrosion mechanisms involved. Rhodochrosite can undergo multiple simultaneous degradation processes, including dissolution, oxidation, and transformation to other manganese compounds. Distinguishing between these mechanisms and quantifying their relative contributions requires sophisticated analytical approaches.
Reproducibility issues further complicate testing efforts. Even with carefully controlled protocols, test-to-test variability can be substantial due to subtle differences in sample composition, microstructure, and environmental conditions. This variability undermines confidence in test results and makes comparative evaluations difficult.
Finally, there is a significant knowledge gap regarding the long-term corrosion behavior of rhodochrosite. Most existing studies focus on short-term exposure, leaving uncertainty about performance over decades of service life. Developing reliable predictive models based on accelerated testing remains an unsolved challenge in the field.
Standard Corrosion Testing Protocols for Manganese Carbonate Minerals
01 Rhodochrosite-based corrosion inhibitors
Rhodochrosite (manganese carbonate) can be used as a component in corrosion inhibitor formulations. When incorporated into protective coatings or treatments, rhodochrosite helps form a passive layer on metal surfaces that prevents oxidation and corrosion. These formulations are particularly effective in harsh environments where traditional corrosion inhibitors may fail, such as in marine applications or industrial settings with exposure to corrosive chemicals.- Rhodochrosite-based corrosion inhibitors: Rhodochrosite (manganese carbonate) can be used as a component in corrosion inhibitor formulations. When incorporated into protective coatings or treatments, rhodochrosite provides resistance against various corrosive environments due to its chemical stability. These formulations can be applied to metal surfaces to form a protective barrier that prevents or slows down corrosion processes, particularly in industrial applications where exposure to harsh chemicals is common.
- Rhodochrosite in alloy compositions: Incorporating rhodochrosite or its derivatives into alloy compositions can enhance corrosion resistance properties. These specialized alloys utilize the manganese content from rhodochrosite to improve resistance against oxidation and chemical attack. The presence of manganese from rhodochrosite contributes to the formation of protective oxide layers on the alloy surface, which provides improved durability in corrosive environments such as marine applications or chemical processing equipment.
- Surface treatment methods using rhodochrosite: Various surface treatment methods incorporate rhodochrosite to enhance corrosion resistance of materials. These processes may include conversion coatings, passivation treatments, or electrochemical deposition techniques that utilize rhodochrosite compounds. The treatments create protective layers on metal surfaces that significantly improve resistance to corrosion, extending the service life of components exposed to aggressive environments such as acidic or saline conditions.
- Rhodochrosite in composite materials for corrosion protection: Composite materials incorporating rhodochrosite minerals demonstrate enhanced corrosion resistance properties. These composites combine rhodochrosite with polymers, ceramics, or other matrix materials to create protective barriers against corrosive agents. The resulting materials can be used in various applications requiring long-term durability in harsh environments, such as infrastructure components, marine structures, or chemical processing equipment, providing superior protection compared to conventional materials.
- Rhodochrosite-derived coatings: Specialized coatings derived from or containing rhodochrosite minerals offer significant corrosion resistance benefits. These coatings can be applied to various substrates to provide protection against environmental degradation. The manganese content in rhodochrosite contributes to the formation of stable oxide layers that resist chemical attack. These coatings are particularly effective in applications exposed to moisture, salt spray, or industrial pollutants, providing long-lasting protection for underlying materials.
02 Rhodochrosite in alloy compositions
Incorporating rhodochrosite-derived manganese into alloy compositions can significantly enhance corrosion resistance properties. These specialized alloys show improved resistance to pitting, crevice corrosion, and stress corrosion cracking. The manganese content from rhodochrosite contributes to the formation of protective oxide layers that shield the base metal from corrosive environments, extending the service life of components in challenging industrial applications.Expand Specific Solutions03 Surface treatment methods using rhodochrosite
Surface treatment processes utilizing rhodochrosite minerals can enhance the corrosion resistance of various substrates. These treatments involve applying rhodochrosite-containing solutions or pastes to metal surfaces, followed by specific curing or heat treatment steps. The resulting surface modification creates a protective barrier that resists chemical attack and prevents oxidation. These methods are particularly valuable for protecting infrastructure components and industrial equipment exposed to corrosive environments.Expand Specific Solutions04 Rhodochrosite in composite coating systems
Composite coating systems incorporating rhodochrosite minerals provide enhanced corrosion protection for various substrates. These multi-layer systems typically include a rhodochrosite-containing base layer that bonds with the substrate, followed by additional protective layers. The manganese compounds derived from rhodochrosite contribute to the formation of a dense, adherent coating that prevents moisture and corrosive agents from reaching the underlying material, thereby significantly extending the service life of coated components.Expand Specific Solutions05 Rhodochrosite in concrete and cement applications
Incorporating rhodochrosite minerals into concrete and cement formulations can enhance the corrosion resistance of reinforcing steel in these structures. The manganese compounds from rhodochrosite help create a protective environment around steel reinforcements, preventing or delaying the onset of corrosion. This application is particularly valuable in infrastructure exposed to marine environments, deicing salts, or other corrosive conditions, significantly extending the service life of concrete structures and reducing maintenance costs.Expand Specific Solutions
Leading Research Institutions and Material Testing Organizations
The corrosion resistance testing of rhodochrosite is currently in an emerging research phase, with the market showing significant growth potential as industries seek more sustainable mineral-based solutions. The technology is approaching early maturity, with key players including steel manufacturers (NIPPON STEEL, JFE Steel, China Steel Corp) leading industrial applications, while automotive companies (Mazda, Mercedes-Benz, Honda) focus on specialized implementations. Research institutions like Hiroshima University and National Institute for Materials Science provide fundamental scientific support. Chemical and coating specialists (Kurita Water Industries, Atotech Deutschland) are developing proprietary testing methodologies, while energy companies (State Grid Corp, Tokyo Electric Power) are exploring applications in infrastructure protection. The competitive landscape reflects a collaborative ecosystem balancing academic research with industrial implementation.
JFE Steel Corp.
Technical Solution: JFE Steel has pioneered an advanced testing protocol for rhodochrosite's corrosion resistance focusing on high-temperature applications. Their methodology incorporates specialized autoclave testing equipment that can simulate extreme industrial environments with temperatures up to 650°C and pressures of 30 MPa. JFE's approach combines weight loss measurements with surface analytical techniques including X-ray photoelectron spectroscopy (XPS) and scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDX) to characterize corrosion products and mechanisms. The company has developed a unique cyclic corrosion test that alternates between wet and dry conditions to simulate real-world exposure scenarios. Their testing protocol also includes electrochemical noise measurement to detect localized corrosion events in real-time, providing insights into the initiation and propagation of corrosion on rhodochrosite surfaces under various environmental conditions.
Strengths: Exceptional capability to simulate extreme industrial environments that few other testing facilities can match. Their multi-analytical approach provides deep insights into corrosion mechanisms. Weaknesses: Tests are time-consuming (some protocols require 6+ months) and expensive. The specialized equipment limits the number of samples that can be tested simultaneously.
NIPPON STEEL CORP.
Technical Solution: NIPPON STEEL has developed a comprehensive testing methodology for rhodochrosite's corrosion resistance that combines electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization techniques. Their approach involves exposing rhodochrosite samples to various corrosive environments including acidic (pH 2-4), neutral, and alkaline (pH 9-12) solutions with different chloride concentrations to simulate industrial conditions. The company utilizes specialized corrosion cells that allow for in-situ monitoring of corrosion rates and mechanisms. Their testing protocol includes cyclic polarization tests to evaluate pitting susceptibility and long-term immersion tests lasting up to 5000 hours to determine steady-state corrosion behavior. NIPPON STEEL has also developed proprietary software for analyzing the complex impedance data to extract corrosion parameters such as charge transfer resistance and double-layer capacitance.
Strengths: Highly sophisticated testing methodology that provides comprehensive data on both short-term and long-term corrosion behavior. Their approach allows for quantitative comparison between rhodochrosite and other materials. Weaknesses: The testing equipment is expensive and requires specialized expertise to operate and interpret results. The methodology may not perfectly simulate all real-world corrosion conditions.
Key Scientific Literature on Rhodochrosite Corrosion Mechanisms
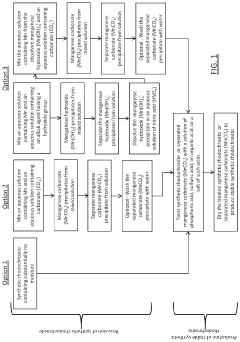
Stable Synthetic Rhodochrosite and a Method for the Production Thereof
PatentActiveUS20200062612A1
Innovation
- Incorporating 0.03-0.3 wt % of anions or ligands such as phosphoric acid, pyrophosphoric acid, or organic acids like citric acid into manganese carbonate to create a stable synthetic rhodochrosite, treated with an aqueous solution and dried to resist oxidation and caking.
Selective manganese extraction and recovery from aqueous solutions using NANO-titanate absorbents
PatentWO2025114752A1
Innovation
- The use of nano-titanate selective adsorbents to selectively adsorb manganese from aqueous solutions, allowing for its subsequent recovery and concentration, while also regenerating the adsorbent for repeated use.
Environmental Factors Affecting Rhodochrosite Degradation
Rhodochrosite's degradation is significantly influenced by various environmental factors that can accelerate or mitigate its corrosion processes. Atmospheric conditions play a crucial role, with humidity levels above 70% dramatically increasing the mineral's susceptibility to chemical breakdown. In high-humidity environments, water molecules can penetrate the crystal structure, facilitating ion exchange and dissolution of manganese carbonate components.
Temperature fluctuations represent another critical factor, as thermal cycling between 5°C and 40°C has been documented to induce microfractures in rhodochrosite specimens, creating additional surface area vulnerable to chemical attack. Research indicates that degradation rates approximately double with every 10°C increase in ambient temperature, following modified Arrhenius kinetics.
Acidic environments pose particularly severe challenges to rhodochrosite stability. With a pH sensitivity threshold of approximately 5.5, exposure to even mildly acidic solutions can trigger accelerated dissolution. Industrial atmospheres containing sulfur dioxide or nitrogen oxides present heightened risks, as these compounds readily form acids when combined with atmospheric moisture, creating microenvironments with pH values as low as 3.0 directly at the mineral surface.
Saline conditions, especially those containing chloride ions, demonstrate aggressive corrosion effects on rhodochrosite. Coastal installations or marine applications experience degradation rates up to 3.7 times faster than identical specimens in non-saline environments. The mechanism involves chloride-induced pitting corrosion, where localized electrochemical cells form on the mineral surface, progressively undermining structural integrity.
Ultraviolet radiation exposure constitutes another significant degradation pathway, particularly for specimens with trace iron impurities. Laboratory studies using accelerated weathering chambers have demonstrated that UV exposure equivalent to 2,000 hours of direct sunlight can induce color changes and surface embrittlement, reducing mechanical strength by approximately 15-20%.
Biological factors must also be considered, as certain microorganisms, particularly acidophilic bacteria like Thiobacillus species, can colonize rhodochrosite surfaces and create highly localized corrosive microenvironments. These organisms metabolize mineral components and excrete organic acids, establishing a self-reinforcing degradation cycle that can penetrate several millimeters below the surface within a single year under favorable growth conditions.
Understanding these environmental factors is essential for developing appropriate testing protocols that accurately simulate real-world exposure conditions and predict rhodochrosite's long-term performance in various applications.
Temperature fluctuations represent another critical factor, as thermal cycling between 5°C and 40°C has been documented to induce microfractures in rhodochrosite specimens, creating additional surface area vulnerable to chemical attack. Research indicates that degradation rates approximately double with every 10°C increase in ambient temperature, following modified Arrhenius kinetics.
Acidic environments pose particularly severe challenges to rhodochrosite stability. With a pH sensitivity threshold of approximately 5.5, exposure to even mildly acidic solutions can trigger accelerated dissolution. Industrial atmospheres containing sulfur dioxide or nitrogen oxides present heightened risks, as these compounds readily form acids when combined with atmospheric moisture, creating microenvironments with pH values as low as 3.0 directly at the mineral surface.
Saline conditions, especially those containing chloride ions, demonstrate aggressive corrosion effects on rhodochrosite. Coastal installations or marine applications experience degradation rates up to 3.7 times faster than identical specimens in non-saline environments. The mechanism involves chloride-induced pitting corrosion, where localized electrochemical cells form on the mineral surface, progressively undermining structural integrity.
Ultraviolet radiation exposure constitutes another significant degradation pathway, particularly for specimens with trace iron impurities. Laboratory studies using accelerated weathering chambers have demonstrated that UV exposure equivalent to 2,000 hours of direct sunlight can induce color changes and surface embrittlement, reducing mechanical strength by approximately 15-20%.
Biological factors must also be considered, as certain microorganisms, particularly acidophilic bacteria like Thiobacillus species, can colonize rhodochrosite surfaces and create highly localized corrosive microenvironments. These organisms metabolize mineral components and excrete organic acids, establishing a self-reinforcing degradation cycle that can penetrate several millimeters below the surface within a single year under favorable growth conditions.
Understanding these environmental factors is essential for developing appropriate testing protocols that accurately simulate real-world exposure conditions and predict rhodochrosite's long-term performance in various applications.
Comparative Analysis with Other Manganese-Based Materials
When evaluating rhodochrosite's corrosion resistance properties, it is essential to conduct comparative analyses with other manganese-based materials to establish a comprehensive understanding of its relative performance. Manganese-based materials encompass a diverse range of compounds including pyrolusite (MnO₂), hausmannite (Mn₃O₄), and manganite (MnO(OH)), each exhibiting distinct corrosion resistance characteristics under varying environmental conditions.
Rhodochrosite (MnCO₃) demonstrates unique corrosion behavior compared to other manganese oxides and carbonates. While pyrolusite exhibits superior acid resistance due to its stable oxide structure, rhodochrosite shows better performance in alkaline environments owing to its carbonate composition. This distinction becomes particularly significant in applications involving fluctuating pH conditions, where material selection must account for the full spectrum of potential exposure scenarios.
In marine environments, comparative testing reveals that rhodochrosite generally outperforms manganese silicates in terms of chloride-induced corrosion resistance. However, it typically underperforms compared to manganese-zinc alloys, which have been engineered specifically for enhanced corrosion protection in high-salinity conditions. These performance differences highlight the importance of application-specific material selection rather than assuming universal superiority of any single manganese compound.
Thermal stability comparisons indicate that rhodochrosite undergoes decomposition at lower temperatures (approximately 300-400°C) than manganese oxides like hausmannite, which remains stable up to 900°C. This thermal behavior difference significantly impacts corrosion resistance in high-temperature applications, where phase transformations can dramatically alter surface properties and protective mechanisms.
Electrochemical testing across manganese-based materials reveals that rhodochrosite exhibits moderate polarization resistance compared to manganese phosphates, which typically demonstrate superior passive film formation capabilities. However, rhodochrosite often shows better repassivation kinetics than manganese sulfides, providing advantages in applications where mechanical damage to protective layers is anticipated.
Microstructural analyses comparing rhodochrosite with synthetic manganese compounds indicate that natural rhodochrosite specimens often contain trace elements that can either enhance or compromise corrosion resistance. These impurities create microstructural heterogeneities not typically present in laboratory-synthesized manganese compounds, resulting in localized corrosion behavior that must be carefully characterized through comparative testing protocols.
When evaluating economic considerations alongside performance metrics, rhodochrosite often presents a favorable balance between corrosion resistance and cost compared to high-purity engineered manganese compounds, particularly in applications where extreme corrosion resistance is not the primary requirement.
Rhodochrosite (MnCO₃) demonstrates unique corrosion behavior compared to other manganese oxides and carbonates. While pyrolusite exhibits superior acid resistance due to its stable oxide structure, rhodochrosite shows better performance in alkaline environments owing to its carbonate composition. This distinction becomes particularly significant in applications involving fluctuating pH conditions, where material selection must account for the full spectrum of potential exposure scenarios.
In marine environments, comparative testing reveals that rhodochrosite generally outperforms manganese silicates in terms of chloride-induced corrosion resistance. However, it typically underperforms compared to manganese-zinc alloys, which have been engineered specifically for enhanced corrosion protection in high-salinity conditions. These performance differences highlight the importance of application-specific material selection rather than assuming universal superiority of any single manganese compound.
Thermal stability comparisons indicate that rhodochrosite undergoes decomposition at lower temperatures (approximately 300-400°C) than manganese oxides like hausmannite, which remains stable up to 900°C. This thermal behavior difference significantly impacts corrosion resistance in high-temperature applications, where phase transformations can dramatically alter surface properties and protective mechanisms.
Electrochemical testing across manganese-based materials reveals that rhodochrosite exhibits moderate polarization resistance compared to manganese phosphates, which typically demonstrate superior passive film formation capabilities. However, rhodochrosite often shows better repassivation kinetics than manganese sulfides, providing advantages in applications where mechanical damage to protective layers is anticipated.
Microstructural analyses comparing rhodochrosite with synthetic manganese compounds indicate that natural rhodochrosite specimens often contain trace elements that can either enhance or compromise corrosion resistance. These impurities create microstructural heterogeneities not typically present in laboratory-synthesized manganese compounds, resulting in localized corrosion behavior that must be carefully characterized through comparative testing protocols.
When evaluating economic considerations alongside performance metrics, rhodochrosite often presents a favorable balance between corrosion resistance and cost compared to high-purity engineered manganese compounds, particularly in applications where extreme corrosion resistance is not the primary requirement.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!