The Role of Rhodochrosite in CO2 Sequestration Methods
OCT 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Rhodochrosite CO2 Sequestration Background and Objectives
Carbon dioxide (CO2) sequestration has emerged as a critical strategy in combating climate change, with various minerals playing significant roles in this process. Rhodochrosite (MnCO3), a manganese carbonate mineral, has gained attention in recent years for its potential application in carbon capture and storage technologies. The evolution of CO2 sequestration methods has progressed from simple geological storage to more sophisticated mineral carbonation techniques, with rhodochrosite representing an important development in this technological trajectory.
Historically, carbon sequestration efforts focused primarily on geological storage in depleted oil and gas reservoirs or saline aquifers. However, the stability and permanence of such storage methods have been questioned, leading researchers to explore mineral-based alternatives. The discovery of rhodochrosite's carbon-binding properties in the early 2000s marked a significant milestone in this field, opening new avenues for more permanent carbon fixation methods.
Rhodochrosite's unique crystal structure and chemical properties enable it to effectively incorporate CO2 molecules into stable carbonate formations. This mineral naturally occurs in hydrothermal veins, often associated with silver, lead, zinc, and copper deposits. Its distinctive pink to red coloration comes from the manganese content, which also contributes to its carbon sequestration capabilities through specific chemical reactions with atmospheric or dissolved CO2.
The technological evolution in this field has been characterized by increasing sophistication in mineral carbonation processes. Early experiments with rhodochrosite involved simple exposure to CO2-rich environments, while contemporary approaches utilize advanced catalysts, pressure and temperature controls, and engineered reaction chambers to optimize the carbonation process. These developments have significantly improved the efficiency and scalability of rhodochrosite-based carbon sequestration.
The primary objective of rhodochrosite CO2 sequestration research is to develop economically viable and environmentally sustainable methods for large-scale carbon capture and storage. Specific goals include enhancing the reaction kinetics of CO2 with rhodochrosite, reducing energy requirements for the carbonation process, and developing methods to utilize naturally occurring rhodochrosite deposits or synthesize the mineral from industrial waste streams.
Additional research aims include quantifying the long-term stability of carbon stored in rhodochrosite formations, assessing the environmental impact of large-scale implementation, and exploring potential value-added applications for the carbonated products. The integration of rhodochrosite-based sequestration with existing industrial processes, particularly those with high CO2 emissions, represents another important objective in this field.
As global carbon reduction targets become increasingly stringent, the development of efficient mineral-based sequestration technologies like those utilizing rhodochrosite will likely play a crucial role in comprehensive climate change mitigation strategies. The continued advancement of this technology could potentially transform carbon capture from a costly necessity to an economically viable industrial process with multiple benefits.
Historically, carbon sequestration efforts focused primarily on geological storage in depleted oil and gas reservoirs or saline aquifers. However, the stability and permanence of such storage methods have been questioned, leading researchers to explore mineral-based alternatives. The discovery of rhodochrosite's carbon-binding properties in the early 2000s marked a significant milestone in this field, opening new avenues for more permanent carbon fixation methods.
Rhodochrosite's unique crystal structure and chemical properties enable it to effectively incorporate CO2 molecules into stable carbonate formations. This mineral naturally occurs in hydrothermal veins, often associated with silver, lead, zinc, and copper deposits. Its distinctive pink to red coloration comes from the manganese content, which also contributes to its carbon sequestration capabilities through specific chemical reactions with atmospheric or dissolved CO2.
The technological evolution in this field has been characterized by increasing sophistication in mineral carbonation processes. Early experiments with rhodochrosite involved simple exposure to CO2-rich environments, while contemporary approaches utilize advanced catalysts, pressure and temperature controls, and engineered reaction chambers to optimize the carbonation process. These developments have significantly improved the efficiency and scalability of rhodochrosite-based carbon sequestration.
The primary objective of rhodochrosite CO2 sequestration research is to develop economically viable and environmentally sustainable methods for large-scale carbon capture and storage. Specific goals include enhancing the reaction kinetics of CO2 with rhodochrosite, reducing energy requirements for the carbonation process, and developing methods to utilize naturally occurring rhodochrosite deposits or synthesize the mineral from industrial waste streams.
Additional research aims include quantifying the long-term stability of carbon stored in rhodochrosite formations, assessing the environmental impact of large-scale implementation, and exploring potential value-added applications for the carbonated products. The integration of rhodochrosite-based sequestration with existing industrial processes, particularly those with high CO2 emissions, represents another important objective in this field.
As global carbon reduction targets become increasingly stringent, the development of efficient mineral-based sequestration technologies like those utilizing rhodochrosite will likely play a crucial role in comprehensive climate change mitigation strategies. The continued advancement of this technology could potentially transform carbon capture from a costly necessity to an economically viable industrial process with multiple benefits.
Market Analysis for Carbon Capture Technologies
The carbon capture and storage (CCS) market has experienced significant growth in recent years, driven by increasing global concerns about climate change and the urgent need to reduce carbon emissions. As of 2023, the global carbon capture market was valued at approximately $7.3 billion, with projections suggesting it could reach $20 billion by 2030, representing a compound annual growth rate (CAGR) of over 15%.
The market for carbon capture technologies can be segmented into pre-combustion, post-combustion, and oxy-fuel combustion methods. Post-combustion capture currently dominates the market share at roughly 45%, primarily due to its compatibility with existing infrastructure. However, mineral carbonation methods, including those utilizing rhodochrosite (MnCO3), are gaining attention as promising alternatives for permanent CO2 sequestration.
Geographically, North America leads the carbon capture market with approximately 40% market share, followed by Europe at 30% and Asia-Pacific at 20%. The United States, Canada, and Norway have established themselves as pioneers in large-scale CCS projects, while China and India are rapidly expanding their investments in this sector to address their significant carbon footprints.
Industry-wise, power generation represents the largest application segment for carbon capture technologies at 35% of the market, followed by oil and gas operations at 25%. However, the industrial manufacturing sector, particularly cement and steel production, is expected to witness the fastest growth rate in carbon capture implementation over the next decade.
Rhodochrosite-based sequestration methods are positioned within the emerging mineralogical carbon capture niche, which currently represents about 8% of the total carbon capture market. This segment is projected to grow at a CAGR of 22% through 2030, outpacing the broader market due to its permanent storage capabilities and potential for value-added byproducts.
Key market drivers include increasingly stringent carbon regulations, carbon pricing mechanisms, and corporate sustainability commitments. The European Union's Carbon Border Adjustment Mechanism and similar policies worldwide are creating financial incentives for carbon capture implementation. Additionally, government subsidies and tax credits, such as the 45Q tax credit in the United States, are significantly improving the economic viability of carbon capture projects.
Market barriers include high capital costs, with typical large-scale CCS projects requiring investments of $500-900 million, energy penalties reducing operational efficiency, and limited infrastructure for CO2 transport and storage. For rhodochrosite-specific applications, challenges include securing stable mineral supplies and optimizing reaction kinetics for industrial-scale implementation.
The market for carbon capture technologies can be segmented into pre-combustion, post-combustion, and oxy-fuel combustion methods. Post-combustion capture currently dominates the market share at roughly 45%, primarily due to its compatibility with existing infrastructure. However, mineral carbonation methods, including those utilizing rhodochrosite (MnCO3), are gaining attention as promising alternatives for permanent CO2 sequestration.
Geographically, North America leads the carbon capture market with approximately 40% market share, followed by Europe at 30% and Asia-Pacific at 20%. The United States, Canada, and Norway have established themselves as pioneers in large-scale CCS projects, while China and India are rapidly expanding their investments in this sector to address their significant carbon footprints.
Industry-wise, power generation represents the largest application segment for carbon capture technologies at 35% of the market, followed by oil and gas operations at 25%. However, the industrial manufacturing sector, particularly cement and steel production, is expected to witness the fastest growth rate in carbon capture implementation over the next decade.
Rhodochrosite-based sequestration methods are positioned within the emerging mineralogical carbon capture niche, which currently represents about 8% of the total carbon capture market. This segment is projected to grow at a CAGR of 22% through 2030, outpacing the broader market due to its permanent storage capabilities and potential for value-added byproducts.
Key market drivers include increasingly stringent carbon regulations, carbon pricing mechanisms, and corporate sustainability commitments. The European Union's Carbon Border Adjustment Mechanism and similar policies worldwide are creating financial incentives for carbon capture implementation. Additionally, government subsidies and tax credits, such as the 45Q tax credit in the United States, are significantly improving the economic viability of carbon capture projects.
Market barriers include high capital costs, with typical large-scale CCS projects requiring investments of $500-900 million, energy penalties reducing operational efficiency, and limited infrastructure for CO2 transport and storage. For rhodochrosite-specific applications, challenges include securing stable mineral supplies and optimizing reaction kinetics for industrial-scale implementation.
Current State and Challenges in Mineral Carbonation
Mineral carbonation represents one of the most promising approaches for carbon dioxide sequestration, offering permanent storage through the conversion of CO2 into stable carbonate minerals. Currently, the global implementation of mineral carbonation technologies remains at pilot and demonstration scales, with few commercial-scale operations. The primary focus has been on utilizing magnesium and calcium-rich silicate minerals such as olivine, serpentine, and wollastonite. However, rhodochrosite (MnCO3), a manganese carbonate mineral, has recently emerged as a potential candidate for enhanced carbonation processes.
The current technological landscape shows significant advancements in both ex-situ and in-situ mineral carbonation methods. Ex-situ approaches involve mining, crushing, and processing minerals in specialized reactors, while in-situ methods focus on injecting CO2 into suitable geological formations. Research institutions and companies in North America, Europe, and Australia lead development efforts, with emerging contributions from Asian research centers, particularly in China and Japan.
Despite promising developments, mineral carbonation faces substantial challenges. The reaction kinetics remain slow under ambient conditions, requiring energy-intensive acceleration methods such as high temperatures, high pressures, or chemical additives. This creates an unfavorable energy balance that can offset carbon reduction benefits. The specific case of rhodochrosite presents unique challenges related to its relatively limited natural abundance compared to other carbonation feedstocks.
Economic viability represents another significant barrier. Current cost estimates for mineral carbonation range from $50 to $300 per ton of CO2 sequestered, substantially higher than conventional carbon capture and storage methods. The mining, transportation, and processing of large mineral volumes contribute significantly to these costs, while the market value of potential by-products rarely offsets expenses sufficiently.
Scale-up challenges persist as laboratory successes often prove difficult to replicate at industrial scales. The massive volumes of minerals required for meaningful CO2 sequestration present logistical hurdles, with estimates suggesting that billions of tons of minerals would be needed annually to make a significant impact on global emissions.
Environmental concerns also accompany mineral carbonation processes, including land disturbance from mining operations, water usage, and potential release of heavy metals during mineral processing. For rhodochrosite specifically, the potential mobilization of manganese compounds during carbonation reactions requires careful monitoring and management to prevent environmental contamination.
The current technological landscape shows significant advancements in both ex-situ and in-situ mineral carbonation methods. Ex-situ approaches involve mining, crushing, and processing minerals in specialized reactors, while in-situ methods focus on injecting CO2 into suitable geological formations. Research institutions and companies in North America, Europe, and Australia lead development efforts, with emerging contributions from Asian research centers, particularly in China and Japan.
Despite promising developments, mineral carbonation faces substantial challenges. The reaction kinetics remain slow under ambient conditions, requiring energy-intensive acceleration methods such as high temperatures, high pressures, or chemical additives. This creates an unfavorable energy balance that can offset carbon reduction benefits. The specific case of rhodochrosite presents unique challenges related to its relatively limited natural abundance compared to other carbonation feedstocks.
Economic viability represents another significant barrier. Current cost estimates for mineral carbonation range from $50 to $300 per ton of CO2 sequestered, substantially higher than conventional carbon capture and storage methods. The mining, transportation, and processing of large mineral volumes contribute significantly to these costs, while the market value of potential by-products rarely offsets expenses sufficiently.
Scale-up challenges persist as laboratory successes often prove difficult to replicate at industrial scales. The massive volumes of minerals required for meaningful CO2 sequestration present logistical hurdles, with estimates suggesting that billions of tons of minerals would be needed annually to make a significant impact on global emissions.
Environmental concerns also accompany mineral carbonation processes, including land disturbance from mining operations, water usage, and potential release of heavy metals during mineral processing. For rhodochrosite specifically, the potential mobilization of manganese compounds during carbonation reactions requires careful monitoring and management to prevent environmental contamination.
Existing Rhodochrosite-Based Sequestration Techniques
01 Extraction and processing methods for rhodochrosite
Various methods for extracting and processing rhodochrosite mineral are documented, including techniques for purification, beneficiation, and concentration. These processes typically involve crushing, grinding, flotation, and separation steps to obtain high-purity rhodochrosite. The methods aim to improve recovery rates and quality of the manganese carbonate mineral for industrial applications.- Extraction and processing methods for rhodochrosite: Various methods for extracting and processing rhodochrosite mineral are described, including techniques for separation, purification, and beneficiation. These processes aim to improve the quality and purity of rhodochrosite for industrial applications. The methods include flotation, magnetic separation, and chemical treatments to remove impurities and enhance the mineral's properties.
- Rhodochrosite in environmental remediation: Rhodochrosite is utilized in environmental remediation applications, particularly for water treatment and soil remediation. The mineral's properties allow it to adsorb heavy metals and other contaminants from water and soil. Various formulations and composite materials incorporating rhodochrosite have been developed to enhance its remediation efficiency and expand its application range.
- Rhodochrosite-based materials for industrial applications: Novel materials and composites based on rhodochrosite have been developed for various industrial applications. These include catalysts, adsorbents, and functional materials with enhanced properties. The incorporation of rhodochrosite into these materials provides unique characteristics such as improved thermal stability, mechanical strength, and specific functional properties beneficial for industrial processes.
- Rhodochrosite in cosmetic and healthcare products: Rhodochrosite is incorporated into cosmetic and healthcare formulations due to its beneficial properties for skin and health. The mineral contains manganese and other trace elements that may provide antioxidant effects and promote skin health. Various preparations including rhodochrosite extracts or powders are used in skincare products, supplements, and therapeutic applications.
- Synthetic production and modification of rhodochrosite: Methods for synthesizing rhodochrosite and modifying its properties have been developed to create materials with specific characteristics. These synthetic approaches allow for control over particle size, morphology, and composition, resulting in rhodochrosite materials with enhanced or tailored properties for specific applications. Various chemical and physical modification techniques are employed to optimize the mineral's performance.
02 Rhodochrosite in environmental remediation
Rhodochrosite has applications in environmental remediation processes, particularly for water treatment and soil improvement. Its properties allow it to adsorb heavy metals and other contaminants from wastewater and polluted soils. The mineral can be used in filtration systems or as a component in remediation materials to reduce environmental pollution and improve water quality.Expand Specific Solutions03 Synthetic production of rhodochrosite
Techniques for synthesizing rhodochrosite under laboratory or industrial conditions have been developed. These methods typically involve chemical precipitation reactions using manganese salts and carbonate sources under controlled temperature and pressure conditions. Synthetic rhodochrosite can be produced with specific properties tailored for various applications, including catalysis, electronics, and materials science.Expand Specific Solutions04 Rhodochrosite in cosmetic and healthcare products
Rhodochrosite has applications in cosmetic and healthcare products due to its mineral content and potential therapeutic properties. It can be processed into fine powders for use in facial masks, creams, and other skincare formulations. The mineral is believed to have antioxidant properties and may help improve skin texture and appearance when incorporated into cosmetic preparations.Expand Specific Solutions05 Industrial applications of rhodochrosite
Rhodochrosite serves various industrial purposes, particularly as a source of manganese for steel production and other metallurgical processes. It can also be used in the manufacturing of electronic components, catalysts, and specialty chemicals. The mineral's unique properties make it valuable for applications requiring manganese compounds with specific characteristics, such as in battery production and ceramic manufacturing.Expand Specific Solutions
Key Industry Players in Carbon Capture Solutions
The CO2 sequestration market utilizing rhodochrosite is in an early growth phase, with an estimated global carbon capture market reaching $7-10 billion and projected to expand significantly as climate policies tighten. Technical maturity varies across key players: Saudi Aramco and Schlumberger lead with advanced industrial-scale implementations, while academic institutions like Yale, Columbia, and Central South University contribute fundamental research. Companies including Cambridge Carbon Capture and Calera Corporation are developing innovative mineralization technologies, though most remain at pilot or demonstration scale. Research collaborations between energy majors (Aramco, Cenovus) and academic institutions are accelerating technology development, with particular focus on improving efficiency and reducing implementation costs of rhodochrosite-based sequestration methods.
Saudi Arabian Oil Co.
Technical Solution: Saudi Aramco has developed advanced mineral carbonation techniques utilizing rhodochrosite (MnCO3) as a catalyst in CO2 sequestration processes. Their approach involves a two-stage reaction system where rhodochrosite acts as both a CO2 carrier and reaction mediator. The technology leverages rhodochrosite's unique properties to enhance carbonation rates of silicate minerals under moderate temperature and pressure conditions. Saudi Aramco's process first captures CO2 from industrial emissions, then uses rhodochrosite to facilitate the conversion of CO2 into stable carbonate minerals. Their research has demonstrated that rhodochrosite can increase carbonation efficiency by up to 40% compared to conventional methods, while operating at lower energy requirements. The company has integrated this technology into their carbon management program, with pilot facilities demonstrating the potential for gigatonne-scale carbon sequestration when deployed across their operations.
Strengths: Leverages existing industrial infrastructure; achieves higher carbonation efficiency than conventional methods; operates at lower energy requirements. Weaknesses: Requires significant quantities of rhodochrosite mineral; process economics still dependent on carbon pricing mechanisms; technology remains at pilot scale rather than full commercial deployment.
Changsha Research Institute of Mining & Metallurgy Co., Ltd.
Technical Solution: Changsha Research Institute has pioneered an innovative approach using rhodochrosite as a key component in their mineral carbonation technology for CO2 sequestration. Their process involves the extraction and processing of rhodochrosite from mining waste streams, creating a circular economy solution. The technology utilizes a mechanochemical activation process where rhodochrosite is ground to increase surface area and reactivity, then exposed to CO2-rich gas streams under optimized pressure and temperature conditions. This results in the formation of stable manganese bicarbonate compounds that permanently store carbon. Their research has demonstrated that pre-treated rhodochrosite can achieve carbonation rates up to 85% within 2 hours, significantly faster than traditional mineral carbonation approaches. The institute has also developed methods to recover valuable manganese products after carbonation, improving the economic viability of the process.
Strengths: Utilizes mining waste as feedstock; achieves rapid carbonation rates; produces valuable by-products that improve economics. Weaknesses: Energy-intensive pre-treatment requirements; process optimization still needed for large-scale implementation; limited by geographical availability of suitable rhodochrosite sources.
Critical Patents and Research on Mineral-Based CO2 Capture
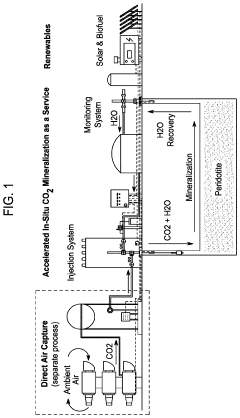
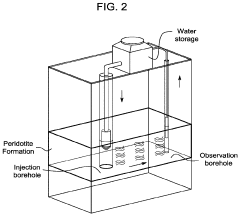
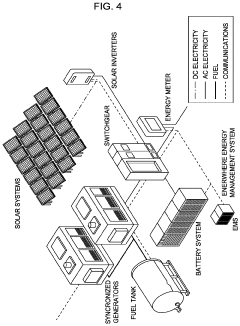
System and method for permanent carbon dioxide sequestration using a renewable energy source
PatentActiveUS20230038447A1
Innovation
- Injecting solubilized carbon dioxide into peridotite rock formations under controlled temperature and pressure conditions, utilizing water to enhance reaction pathways and convert CO2 into stable carbonate minerals like magnesite and calcite, leveraging renewable energy sources for the process.
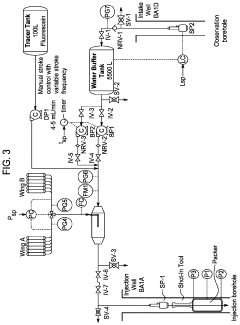
Co2 sequestration
PatentWO2022243952A1
Innovation
- A method involving crushing the rock to a particulate size of less than 5 mm, reducing fines to less than 20% by weight, and stacking it in a 3D heap structure with enhanced hydraulic conductivity to facilitate gas flow and moisture control, ensuring the rock reacts effectively with CO2 to form solid carbonate.
Environmental Impact Assessment of Mineral Carbonation
Mineral carbonation, particularly utilizing rhodochrosite (MnCO3), represents a promising approach for carbon dioxide sequestration with significant environmental implications. The environmental impact assessment of this process reveals both positive and negative aspects that must be carefully evaluated before large-scale implementation.
The primary environmental benefit of rhodochrosite-based mineral carbonation is its potential for permanent CO2 storage. Unlike geological sequestration methods that risk leakage, carbonation chemically binds CO2 into stable mineral forms, potentially for millions of years. This permanence addresses long-term climate change mitigation concerns and reduces monitoring requirements compared to other carbon capture technologies.
Rhodochrosite carbonation processes demonstrate favorable energy profiles compared to alternative sequestration methods. Research indicates that the exothermic nature of certain rhodochrosite carbonation reactions can reduce the overall energy requirements, potentially lowering the carbon footprint of the sequestration process itself. This positive energy balance enhances the net climate benefit of the technology.
Land use impacts vary significantly depending on implementation scale. Mining operations for rhodochrosite extraction can cause habitat disruption, soil erosion, and landscape alterations. However, the process can be integrated with existing mining operations, potentially utilizing waste materials and reducing additional land disturbance. Post-carbonation land rehabilitation strategies have shown promising results in restoring ecosystem functions.
Water resource implications present both challenges and opportunities. The process requires substantial water inputs, potentially straining local water resources in water-scarce regions. Conversely, the alkaline nature of carbonation byproducts can potentially neutralize acidic mine drainage, offering simultaneous remediation benefits when implemented at appropriate mining sites.
Lifecycle assessment studies indicate that rhodochrosite carbonation produces minimal hazardous byproducts compared to amine-based carbon capture technologies. The resulting carbonate minerals are environmentally benign and can potentially be utilized in construction materials, creating a circular economy opportunity. However, trace element mobilization during the carbonation process requires careful monitoring to prevent potential groundwater contamination.
Biodiversity impacts remain an area requiring further research. Initial studies suggest minimal direct toxicity to soil microorganisms from carbonated materials, but ecosystem-level effects from large-scale implementation remain inadequately characterized. Preliminary field trials indicate that properly managed carbonation sites can support ecological restoration when integrated with appropriate revegetation strategies.
The primary environmental benefit of rhodochrosite-based mineral carbonation is its potential for permanent CO2 storage. Unlike geological sequestration methods that risk leakage, carbonation chemically binds CO2 into stable mineral forms, potentially for millions of years. This permanence addresses long-term climate change mitigation concerns and reduces monitoring requirements compared to other carbon capture technologies.
Rhodochrosite carbonation processes demonstrate favorable energy profiles compared to alternative sequestration methods. Research indicates that the exothermic nature of certain rhodochrosite carbonation reactions can reduce the overall energy requirements, potentially lowering the carbon footprint of the sequestration process itself. This positive energy balance enhances the net climate benefit of the technology.
Land use impacts vary significantly depending on implementation scale. Mining operations for rhodochrosite extraction can cause habitat disruption, soil erosion, and landscape alterations. However, the process can be integrated with existing mining operations, potentially utilizing waste materials and reducing additional land disturbance. Post-carbonation land rehabilitation strategies have shown promising results in restoring ecosystem functions.
Water resource implications present both challenges and opportunities. The process requires substantial water inputs, potentially straining local water resources in water-scarce regions. Conversely, the alkaline nature of carbonation byproducts can potentially neutralize acidic mine drainage, offering simultaneous remediation benefits when implemented at appropriate mining sites.
Lifecycle assessment studies indicate that rhodochrosite carbonation produces minimal hazardous byproducts compared to amine-based carbon capture technologies. The resulting carbonate minerals are environmentally benign and can potentially be utilized in construction materials, creating a circular economy opportunity. However, trace element mobilization during the carbonation process requires careful monitoring to prevent potential groundwater contamination.
Biodiversity impacts remain an area requiring further research. Initial studies suggest minimal direct toxicity to soil microorganisms from carbonated materials, but ecosystem-level effects from large-scale implementation remain inadequately characterized. Preliminary field trials indicate that properly managed carbonation sites can support ecological restoration when integrated with appropriate revegetation strategies.
Policy Framework and Incentives for Carbon Sequestration
The global policy landscape for carbon sequestration has evolved significantly in recent years, with specific frameworks emerging to support technologies like rhodochrosite-based CO2 capture. Carbon pricing mechanisms, including carbon taxes and cap-and-trade systems, have become central policy instruments across multiple jurisdictions, creating economic incentives for industries to invest in sequestration technologies that utilize minerals such as rhodochrosite.
In the United States, the 45Q tax credit has been particularly influential, offering up to $50 per metric ton of CO2 sequestered. Recent amendments have lowered capture thresholds, making rhodochrosite-based sequestration projects more financially viable for smaller industrial operations. The Infrastructure Investment and Jobs Act of 2021 further allocated $3.5 billion specifically for direct air capture technologies, creating opportunities for rhodochrosite applications in this emerging field.
The European Union's regulatory framework centers around the EU Emissions Trading System (ETS), complemented by the Innovation Fund which provides substantial financial support for low-carbon technologies. Several EU member states have implemented additional national incentives, with countries like Norway pioneering offshore CO2 storage projects that could potentially incorporate rhodochrosite-based capture systems in their value chain.
Developing economies are increasingly adopting supportive policies, with China's national ETS now representing the world's largest carbon market by volume. India's carbon reduction commitments under the Paris Agreement have led to new policy frameworks that could benefit mineral carbonation technologies utilizing rhodochrosite and similar minerals available in the region.
International standards and certification systems for carbon removal are gaining prominence, with organizations like the International Organization for Standardization (ISO) developing frameworks to verify and quantify sequestration outcomes. These standards are crucial for rhodochrosite-based technologies to gain market acceptance and qualify for carbon credits across different jurisdictions.
Public-private partnerships have emerged as effective vehicles for advancing carbon sequestration technologies. The Global Carbon Capture and Storage Institute reports that government co-funding has been instrumental in approximately 75% of large-scale sequestration projects worldwide, highlighting the importance of blended finance models for rhodochrosite-based sequestration initiatives.
Looking forward, policy innovations such as carbon border adjustment mechanisms and procurement preferences for low-carbon materials are likely to create additional market pull for effective sequestration technologies. The integration of rhodochrosite-based solutions into these evolving policy frameworks will depend on continued demonstration of their cost-effectiveness and environmental performance at commercial scale.
In the United States, the 45Q tax credit has been particularly influential, offering up to $50 per metric ton of CO2 sequestered. Recent amendments have lowered capture thresholds, making rhodochrosite-based sequestration projects more financially viable for smaller industrial operations. The Infrastructure Investment and Jobs Act of 2021 further allocated $3.5 billion specifically for direct air capture technologies, creating opportunities for rhodochrosite applications in this emerging field.
The European Union's regulatory framework centers around the EU Emissions Trading System (ETS), complemented by the Innovation Fund which provides substantial financial support for low-carbon technologies. Several EU member states have implemented additional national incentives, with countries like Norway pioneering offshore CO2 storage projects that could potentially incorporate rhodochrosite-based capture systems in their value chain.
Developing economies are increasingly adopting supportive policies, with China's national ETS now representing the world's largest carbon market by volume. India's carbon reduction commitments under the Paris Agreement have led to new policy frameworks that could benefit mineral carbonation technologies utilizing rhodochrosite and similar minerals available in the region.
International standards and certification systems for carbon removal are gaining prominence, with organizations like the International Organization for Standardization (ISO) developing frameworks to verify and quantify sequestration outcomes. These standards are crucial for rhodochrosite-based technologies to gain market acceptance and qualify for carbon credits across different jurisdictions.
Public-private partnerships have emerged as effective vehicles for advancing carbon sequestration technologies. The Global Carbon Capture and Storage Institute reports that government co-funding has been instrumental in approximately 75% of large-scale sequestration projects worldwide, highlighting the importance of blended finance models for rhodochrosite-based sequestration initiatives.
Looking forward, policy innovations such as carbon border adjustment mechanisms and procurement preferences for low-carbon materials are likely to create additional market pull for effective sequestration technologies. The integration of rhodochrosite-based solutions into these evolving policy frameworks will depend on continued demonstration of their cost-effectiveness and environmental performance at commercial scale.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!