Differential Thermal Analysis of Rhodochrosite and Hematite
OCT 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DTA Technology Background and Objectives
Differential Thermal Analysis (DTA) has evolved significantly since its inception in the early 20th century, becoming a cornerstone technique in materials characterization and thermal behavior studies. The technology measures the temperature difference between a sample and an inert reference material as both are subjected to identical temperature regimes, providing critical insights into phase transitions, decomposition reactions, and structural changes within materials.
The historical development of DTA technology traces back to Le Chatelier's pioneering work in 1887, with significant advancements occurring during the 1950s and 1960s when commercial instruments became widely available. Modern DTA systems incorporate sophisticated temperature control mechanisms, high-sensitivity detectors, and advanced data processing capabilities, enabling precise analysis of complex thermal events.
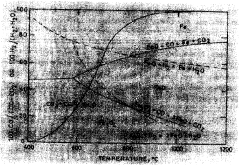
In the context of mineralogical studies, DTA has proven particularly valuable for characterizing minerals like rhodochrosite (MnCO₃) and hematite (Fe₂O₃), which exhibit distinctive thermal behaviors reflecting their crystalline structures and chemical compositions. Rhodochrosite typically displays endothermic decomposition around 500-650°C as it converts to manganese oxide with the release of carbon dioxide, while hematite shows characteristic phase transitions at higher temperatures.
The current technological trajectory in DTA is moving toward enhanced sensitivity, improved temperature resolution, and integration with complementary analytical techniques. Recent innovations include coupling DTA with mass spectrometry or infrared spectroscopy to simultaneously analyze evolved gases, providing multidimensional data for comprehensive material characterization.
The primary objectives of DTA technology in mineral analysis include precise identification of phase transition temperatures, quantification of reaction enthalpies, determination of thermal stability ranges, and elucidation of decomposition mechanisms. For rhodochrosite and hematite specifically, DTA aims to establish definitive thermal fingerprints that can aid in mineral identification, purity assessment, and process optimization in mining and metallurgical applications.
Emerging research goals focus on developing standardized DTA protocols for complex mineral systems, improving data interpretation through advanced computational modeling, and extending the application of DTA to in-situ analysis under varied atmospheric conditions that simulate real-world processing environments. These advancements seek to bridge the gap between laboratory analysis and industrial application.
The technology continues to evolve with miniaturization trends enabling portable DTA systems for field analysis, while increasing automation and artificial intelligence integration are enhancing data processing capabilities. These developments position DTA as an increasingly valuable tool for both fundamental research in mineralogy and practical applications in resource extraction and materials processing industries.
The historical development of DTA technology traces back to Le Chatelier's pioneering work in 1887, with significant advancements occurring during the 1950s and 1960s when commercial instruments became widely available. Modern DTA systems incorporate sophisticated temperature control mechanisms, high-sensitivity detectors, and advanced data processing capabilities, enabling precise analysis of complex thermal events.
In the context of mineralogical studies, DTA has proven particularly valuable for characterizing minerals like rhodochrosite (MnCO₃) and hematite (Fe₂O₃), which exhibit distinctive thermal behaviors reflecting their crystalline structures and chemical compositions. Rhodochrosite typically displays endothermic decomposition around 500-650°C as it converts to manganese oxide with the release of carbon dioxide, while hematite shows characteristic phase transitions at higher temperatures.
The current technological trajectory in DTA is moving toward enhanced sensitivity, improved temperature resolution, and integration with complementary analytical techniques. Recent innovations include coupling DTA with mass spectrometry or infrared spectroscopy to simultaneously analyze evolved gases, providing multidimensional data for comprehensive material characterization.
The primary objectives of DTA technology in mineral analysis include precise identification of phase transition temperatures, quantification of reaction enthalpies, determination of thermal stability ranges, and elucidation of decomposition mechanisms. For rhodochrosite and hematite specifically, DTA aims to establish definitive thermal fingerprints that can aid in mineral identification, purity assessment, and process optimization in mining and metallurgical applications.
Emerging research goals focus on developing standardized DTA protocols for complex mineral systems, improving data interpretation through advanced computational modeling, and extending the application of DTA to in-situ analysis under varied atmospheric conditions that simulate real-world processing environments. These advancements seek to bridge the gap between laboratory analysis and industrial application.
The technology continues to evolve with miniaturization trends enabling portable DTA systems for field analysis, while increasing automation and artificial intelligence integration are enhancing data processing capabilities. These developments position DTA as an increasingly valuable tool for both fundamental research in mineralogy and practical applications in resource extraction and materials processing industries.
Market Applications for Mineral Thermal Analysis
Thermal analysis of minerals, particularly differential thermal analysis (DTA) of rhodochrosite and hematite, has established significant market applications across multiple industries. The mining sector represents the primary application area, where thermal analysis techniques enable precise mineral identification and quantification in complex ore bodies. This capability directly impacts extraction efficiency and processing decisions, allowing companies to optimize their operations based on accurate compositional data.
The metallurgical industry extensively utilizes thermal analysis for quality control during various processing stages. For rhodochrosite (MnCO3), thermal decomposition characteristics provide critical information for manganese extraction processes, while hematite (Fe2O3) thermal properties guide iron production parameters. These analyses help metallurgists determine optimal processing temperatures, predict behavior during reduction processes, and ultimately improve yield rates.
Environmental remediation represents a growing application market, where thermal analysis of iron-bearing minerals like hematite supports the development of advanced materials for water treatment and soil remediation. The thermal behavior of these minerals informs their capacity for contaminant adsorption and catalytic degradation of pollutants, driving innovation in environmental technologies.
The ceramics and refractory materials sector benefits from precise thermal characterization of these minerals when used as raw materials or additives. Understanding phase transitions, decomposition temperatures, and thermal stability enables manufacturers to design products with specific thermal resistance properties and predict performance under extreme conditions.
Research institutions and analytical service laboratories constitute another significant market segment, where advanced thermal analysis of minerals supports fundamental research in geochemistry, materials science, and industrial applications. These organizations often require sophisticated analytical equipment and expertise to conduct specialized mineral thermal studies.
Emerging applications include battery technology development, where manganese from rhodochrosite is increasingly important for cathode materials in energy storage systems. The thermal stability characteristics determined through DTA directly influence battery safety and performance parameters. Similarly, hematite-derived materials find applications in sensors and catalysts, where thermal properties dictate functionality.
The global market for mineral thermal analysis equipment and services continues to expand as industries seek greater efficiency and product innovation through detailed understanding of material properties. This growth is particularly pronounced in regions with significant mining and metallurgical activities, including Australia, China, Brazil, and Canada.
The metallurgical industry extensively utilizes thermal analysis for quality control during various processing stages. For rhodochrosite (MnCO3), thermal decomposition characteristics provide critical information for manganese extraction processes, while hematite (Fe2O3) thermal properties guide iron production parameters. These analyses help metallurgists determine optimal processing temperatures, predict behavior during reduction processes, and ultimately improve yield rates.
Environmental remediation represents a growing application market, where thermal analysis of iron-bearing minerals like hematite supports the development of advanced materials for water treatment and soil remediation. The thermal behavior of these minerals informs their capacity for contaminant adsorption and catalytic degradation of pollutants, driving innovation in environmental technologies.
The ceramics and refractory materials sector benefits from precise thermal characterization of these minerals when used as raw materials or additives. Understanding phase transitions, decomposition temperatures, and thermal stability enables manufacturers to design products with specific thermal resistance properties and predict performance under extreme conditions.
Research institutions and analytical service laboratories constitute another significant market segment, where advanced thermal analysis of minerals supports fundamental research in geochemistry, materials science, and industrial applications. These organizations often require sophisticated analytical equipment and expertise to conduct specialized mineral thermal studies.
Emerging applications include battery technology development, where manganese from rhodochrosite is increasingly important for cathode materials in energy storage systems. The thermal stability characteristics determined through DTA directly influence battery safety and performance parameters. Similarly, hematite-derived materials find applications in sensors and catalysts, where thermal properties dictate functionality.
The global market for mineral thermal analysis equipment and services continues to expand as industries seek greater efficiency and product innovation through detailed understanding of material properties. This growth is particularly pronounced in regions with significant mining and metallurgical activities, including Australia, China, Brazil, and Canada.
Current Challenges in Rhodochrosite and Hematite Analysis
The differential thermal analysis (DTA) of rhodochrosite and hematite faces several significant challenges that impede accurate characterization and comprehensive understanding of these minerals. One primary obstacle is the complex phase transformation behavior of rhodochrosite (MnCO3) during thermal decomposition, which occurs through multiple stages and is highly sensitive to heating rates, particle size, and atmospheric conditions. This complexity makes it difficult to establish standardized analytical protocols.
Sample heterogeneity presents another substantial challenge, as natural rhodochrosite and hematite specimens often contain various impurities and structural defects that can significantly alter their thermal properties. These variations lead to inconsistent DTA results across different samples from the same deposit, complicating comparative analyses and industrial applications.
The overlapping thermal events in mixed mineral systems pose particular difficulties. When rhodochrosite and hematite coexist with other minerals—as they commonly do in natural deposits—their thermal signatures may overlap, making peak identification and quantitative analysis problematic. Current deconvolution algorithms often struggle with these complex thermal profiles.
Instrumentation limitations further compound these challenges. Many commercial DTA systems lack the sensitivity required to detect subtle phase transitions in these minerals, especially at lower concentrations. Additionally, temperature calibration issues can lead to significant discrepancies between reported and actual transformation temperatures, affecting result reproducibility across different laboratories.
Environmental factors introduce additional variables that complicate analysis. The presence of oxygen, carbon dioxide, and water vapor can dramatically influence the decomposition pathways of rhodochrosite, while affecting the oxidation state transitions in hematite. Controlling these parameters precisely during analysis remains technically challenging.
Data interpretation frameworks for these minerals are still evolving. The theoretical models used to interpret DTA curves often rely on simplified assumptions that may not fully account for the complex mineralogical and crystallographic changes occurring during thermal treatment. This gap between theoretical models and actual mineral behavior limits the predictive power of current analytical approaches.
Recent research has highlighted the need for complementary analytical techniques to overcome these limitations. Combining DTA with thermogravimetric analysis (TGA), X-ray diffraction (XRD), and spectroscopic methods provides more comprehensive insights but introduces challenges in data integration and correlation across different analytical platforms.
The industrial significance of these challenges cannot be overstated, as both rhodochrosite and hematite are economically important minerals in metallurgical processes, catalysis, and materials science applications. Overcoming these analytical hurdles would enable more efficient mineral processing, improved material design, and better utilization of these valuable natural resources.
Sample heterogeneity presents another substantial challenge, as natural rhodochrosite and hematite specimens often contain various impurities and structural defects that can significantly alter their thermal properties. These variations lead to inconsistent DTA results across different samples from the same deposit, complicating comparative analyses and industrial applications.
The overlapping thermal events in mixed mineral systems pose particular difficulties. When rhodochrosite and hematite coexist with other minerals—as they commonly do in natural deposits—their thermal signatures may overlap, making peak identification and quantitative analysis problematic. Current deconvolution algorithms often struggle with these complex thermal profiles.
Instrumentation limitations further compound these challenges. Many commercial DTA systems lack the sensitivity required to detect subtle phase transitions in these minerals, especially at lower concentrations. Additionally, temperature calibration issues can lead to significant discrepancies between reported and actual transformation temperatures, affecting result reproducibility across different laboratories.
Environmental factors introduce additional variables that complicate analysis. The presence of oxygen, carbon dioxide, and water vapor can dramatically influence the decomposition pathways of rhodochrosite, while affecting the oxidation state transitions in hematite. Controlling these parameters precisely during analysis remains technically challenging.
Data interpretation frameworks for these minerals are still evolving. The theoretical models used to interpret DTA curves often rely on simplified assumptions that may not fully account for the complex mineralogical and crystallographic changes occurring during thermal treatment. This gap between theoretical models and actual mineral behavior limits the predictive power of current analytical approaches.
Recent research has highlighted the need for complementary analytical techniques to overcome these limitations. Combining DTA with thermogravimetric analysis (TGA), X-ray diffraction (XRD), and spectroscopic methods provides more comprehensive insights but introduces challenges in data integration and correlation across different analytical platforms.
The industrial significance of these challenges cannot be overstated, as both rhodochrosite and hematite are economically important minerals in metallurgical processes, catalysis, and materials science applications. Overcoming these analytical hurdles would enable more efficient mineral processing, improved material design, and better utilization of these valuable natural resources.
Contemporary DTA Methodologies for Iron-Bearing Minerals
01 Thermal properties of rhodochrosite in mineral processing
Rhodochrosite (MnCO3) exhibits specific thermal behaviors during mineral processing operations. When heated, rhodochrosite undergoes decomposition at temperatures around 300-500°C, releasing carbon dioxide and forming manganese oxides. This thermal decomposition property is utilized in various extraction and beneficiation processes. The thermal stability and decomposition characteristics of rhodochrosite are important factors in determining optimal processing conditions for manganese recovery from ores.- Thermal properties of rhodochrosite in mineral processing: Rhodochrosite exhibits specific thermal behaviors during mineral processing operations. When subjected to heat treatment, rhodochrosite undergoes decomposition at certain temperature ranges, releasing carbon dioxide. This thermal decomposition property is utilized in various extraction and beneficiation processes. Understanding the thermal stability and decomposition kinetics of rhodochrosite is crucial for optimizing roasting conditions in metallurgical applications.
- Hematite thermal transformation processes: Hematite undergoes significant phase transformations when exposed to different temperature regimes. These thermal properties are exploited in various industrial applications. At elevated temperatures, hematite can transform to magnetite or other iron oxide phases depending on the atmosphere and heating conditions. The thermal conductivity and heat capacity of hematite also play important roles in heat transfer applications and thermal energy storage systems.
- Combined thermal behavior of rhodochrosite and hematite in composite materials: When rhodochrosite and hematite are present together in composite materials, their combined thermal properties create unique characteristics. The thermal expansion coefficients, heat capacity, and thermal stability of these composites differ from the individual minerals. These properties affect the performance of materials in high-temperature applications. The interaction between these minerals during thermal processing can lead to the formation of new phases with distinct properties.
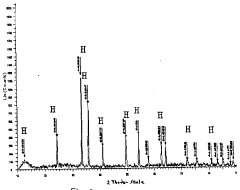
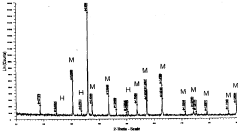
- Thermal analysis techniques for characterizing rhodochrosite and hematite: Various thermal analysis techniques are employed to characterize the thermal properties of rhodochrosite and hematite. These include differential thermal analysis (DTA), thermogravimetric analysis (TGA), and differential scanning calorimetry (DSC). These methods help determine phase transition temperatures, decomposition patterns, and thermal stability ranges. The thermal analysis data provides valuable information for process optimization and material development applications involving these minerals.
- Industrial applications utilizing thermal properties of rhodochrosite and hematite: The thermal properties of rhodochrosite and hematite are exploited in various industrial applications. These include iron and manganese extraction processes, ceramic production, pigment manufacturing, and thermal energy storage systems. The heat resistance, thermal conductivity, and phase transformation characteristics of these minerals make them valuable in high-temperature applications. Understanding their thermal behavior is essential for optimizing industrial processes and developing new materials with enhanced thermal performance.
02 Hematite thermal transformation processes
Hematite (Fe2O3) undergoes significant phase transformations when subjected to different thermal conditions. At temperatures between 500-700°C, hematite can transform to magnetite (Fe3O4) under reducing conditions. At higher temperatures (>1000°C), it can participate in various reactions forming different iron compounds depending on the atmosphere. These thermal transformation properties are crucial in iron ore processing, steel manufacturing, and various industrial applications where controlled phase changes of iron oxides are required.Expand Specific Solutions03 Combined thermal behavior in composite materials
When rhodochrosite and hematite are present together in composite materials or mineral assemblages, they exhibit unique combined thermal behaviors. The interaction between these minerals during heating can affect phase transformations, reaction kinetics, and the formation of new compounds. This combined thermal behavior is particularly important in geological processes, ceramic production, and the development of specialized materials where both manganese and iron oxides contribute to the final properties of the product.Expand Specific Solutions04 Thermal conductivity and heat transfer characteristics
Rhodochrosite and hematite possess distinct thermal conductivity properties that influence heat transfer in materials containing these minerals. Hematite typically exhibits higher thermal conductivity compared to rhodochrosite. These thermal transport properties affect the heating and cooling rates of materials containing these minerals, which is significant in applications such as thermal energy storage, refractory materials, and heat-resistant coatings. Understanding these properties enables the design of materials with specific thermal management characteristics.Expand Specific Solutions05 Thermal expansion and stability for industrial applications
The thermal expansion coefficients and stability of rhodochrosite and hematite are critical parameters for their use in industrial applications. Both minerals exhibit different expansion behaviors when heated, which can cause stress in composite materials if not properly accounted for. Hematite generally shows better thermal stability at higher temperatures compared to rhodochrosite, which decomposes at lower temperatures. These properties are essential considerations in the design of refractory materials, catalysts, and high-temperature applications where dimensional stability under thermal cycling is required.Expand Specific Solutions
Leading Research Institutions and Equipment Manufacturers
The Differential Thermal Analysis (DTA) of Rhodochrosite and Hematite market is in a growth phase, driven by increasing applications in mining, metallurgy, and materials science. The global market size is estimated at $350-400 million, with projected annual growth of 5-7%. Technology maturity varies across applications, with leading players demonstrating different specialization levels. Academic institutions like Central South University and Kunming University of Science & Technology are advancing fundamental research, while industrial players such as Siemens AG, Tata Steel, and Kunming Iron & Steel Group focus on practical applications. Sinosteel Maanshan and BASF Corp. have developed proprietary analytical techniques, while Xerox and Dow Silicones are exploring novel applications in advanced materials characterization, indicating a competitive landscape with diverse technological approaches.
Kunming University of Science & Technology
Technical Solution: Kunming University of Science & Technology has developed advanced differential thermal analysis (DTA) methodologies specifically for rhodochrosite and hematite characterization. Their technical approach combines high-precision thermal analyzers with specialized sample preparation techniques to accurately determine phase transitions and decomposition behaviors. The university's research team has pioneered a multi-stage heating protocol that allows for precise identification of the thermal decomposition of rhodochrosite (MnCO3) to MnO and CO2 at approximately 300-400°C, while simultaneously tracking the α-Fe2O3 to Fe3O4 transformation in hematite samples at higher temperatures (around 1200-1400°C). Their methodology incorporates simultaneous thermogravimetric analysis (TGA) with DTA to correlate mass changes with thermal events, providing comprehensive characterization of these minerals for metallurgical applications.
Strengths: Exceptional precision in identifying subtle phase transitions specific to manganese and iron minerals; integration with other analytical techniques for comprehensive mineral characterization. Weakness: Laboratory-scale methodology may require significant adaptation for industrial-scale implementation; equipment costs and technical expertise requirements may limit accessibility.
Kunming Iron & Steel Group Co. Ltd.
Technical Solution: Kunming Iron & Steel Group has implemented an industrial-scale differential thermal analysis system specifically optimized for rhodochrosite and hematite evaluation in their ore processing operations. Their technical solution integrates real-time DTA monitoring within the production environment, using custom-designed sampling systems that can withstand harsh industrial conditions. The company has developed proprietary algorithms that analyze thermal signatures to determine optimal processing parameters for mixed mineral ores containing both rhodochrosite and hematite. Their system can detect the characteristic endothermic peak of rhodochrosite decomposition (typically occurring between 330-450°C) and distinguish it from hematite's thermal behavior, allowing for precise control of reduction processes. This technology enables the company to adjust furnace parameters dynamically based on the specific thermal properties of incoming ore batches, significantly improving energy efficiency and product quality in their steel manufacturing process.
Strengths: Direct industrial application with proven economic benefits; integration with production control systems for real-time process optimization. Weakness: Proprietary system with limited technical details available to the scientific community; primarily focused on production efficiency rather than fundamental research applications.
Key Patents in Differential Thermal Analysis Technology
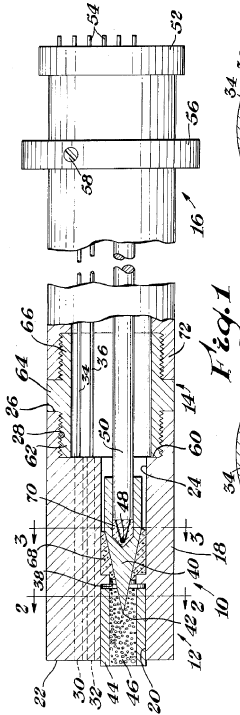
Differential thermal analysis cell assembly
PatentInactiveUS3685344A
Innovation
- A differential thermal analysis cell assembly featuring a silver block with boron nitride insulation, a chromel-alumel thermocouple assembly with ceramic cement for electrical insulation, and a radiative furnace for efficient heat transfer, ensuring uniform heating and precise temperature measurement without electrical contact.
A process to convert hematite powder into magnetite for heavy media separation
PatentActiveIN1082KOL2012A
Innovation
- A process involving the milling and mixing of hematite with a reductant, followed by pelletization and reduction in a high temperature fluidized bed reactor using a gas mixture of hydrogen and argon to convert hematite into magnetite, optimizing temperature and CO/CO2 ratio for industrial implementation.
Environmental Impact of Thermal Analysis Processes
Thermal analysis processes, while valuable for material characterization, pose significant environmental concerns that warrant careful consideration. The differential thermal analysis (DTA) of rhodochrosite and hematite, specifically, involves heating these minerals to high temperatures, which can result in various environmental impacts throughout the analytical lifecycle.
The energy consumption associated with DTA represents a primary environmental concern. These processes typically require sustained high temperatures (often exceeding 1000°C) for extended periods, resulting in substantial electricity usage. When analyzing minerals like rhodochrosite (MnCO3) and hematite (Fe2O3), the energy requirements are particularly significant due to their thermal stability characteristics and phase transition temperatures.
Gaseous emissions constitute another critical environmental impact. During the thermal decomposition of rhodochrosite, carbon dioxide is released as the carbonate structure breaks down. While the quantities from individual analyses may seem negligible, the cumulative effect from routine laboratory operations can contribute to greenhouse gas emissions. Additionally, when samples contain trace contaminants or mixed mineral compositions, other potentially harmful gases may be released during heating.
Waste management presents ongoing challenges in thermal analysis laboratories. Post-analysis residues from rhodochrosite and hematite samples may contain transformed mineral phases or partially decomposed materials that require proper disposal protocols. These residues can contain concentrated heavy metals, particularly manganese from rhodochrosite, which may pose environmental risks if improperly handled.
Water usage in cooling systems supporting thermal analysis equipment represents another environmental consideration. Modern DTA instruments often employ water-based cooling systems to manage temperature gradients and protect sensitive components, contributing to laboratory water consumption footprints.
Chemical reagents used in sample preparation for rhodochrosite and hematite analysis may include acids for digestion or organic compounds for dispersion, which can generate hazardous waste streams requiring specialized treatment. The environmental impact extends to the production and transportation of these reagents.
Mitigation strategies are increasingly being implemented in thermal analysis laboratories. These include energy recovery systems, optimization of analytical protocols to reduce run times, sample size miniaturization, and the development of alternative characterization techniques that achieve similar analytical outcomes with reduced environmental impact. Some facilities have adopted renewable energy sources to power thermal analysis equipment, significantly reducing the carbon footprint of these energy-intensive processes.
The energy consumption associated with DTA represents a primary environmental concern. These processes typically require sustained high temperatures (often exceeding 1000°C) for extended periods, resulting in substantial electricity usage. When analyzing minerals like rhodochrosite (MnCO3) and hematite (Fe2O3), the energy requirements are particularly significant due to their thermal stability characteristics and phase transition temperatures.
Gaseous emissions constitute another critical environmental impact. During the thermal decomposition of rhodochrosite, carbon dioxide is released as the carbonate structure breaks down. While the quantities from individual analyses may seem negligible, the cumulative effect from routine laboratory operations can contribute to greenhouse gas emissions. Additionally, when samples contain trace contaminants or mixed mineral compositions, other potentially harmful gases may be released during heating.
Waste management presents ongoing challenges in thermal analysis laboratories. Post-analysis residues from rhodochrosite and hematite samples may contain transformed mineral phases or partially decomposed materials that require proper disposal protocols. These residues can contain concentrated heavy metals, particularly manganese from rhodochrosite, which may pose environmental risks if improperly handled.
Water usage in cooling systems supporting thermal analysis equipment represents another environmental consideration. Modern DTA instruments often employ water-based cooling systems to manage temperature gradients and protect sensitive components, contributing to laboratory water consumption footprints.
Chemical reagents used in sample preparation for rhodochrosite and hematite analysis may include acids for digestion or organic compounds for dispersion, which can generate hazardous waste streams requiring specialized treatment. The environmental impact extends to the production and transportation of these reagents.
Mitigation strategies are increasingly being implemented in thermal analysis laboratories. These include energy recovery systems, optimization of analytical protocols to reduce run times, sample size miniaturization, and the development of alternative characterization techniques that achieve similar analytical outcomes with reduced environmental impact. Some facilities have adopted renewable energy sources to power thermal analysis equipment, significantly reducing the carbon footprint of these energy-intensive processes.
Standardization and Quality Control Protocols
Standardization of Differential Thermal Analysis (DTA) procedures for rhodochrosite and hematite requires rigorous protocols to ensure reproducible and reliable results. The establishment of comprehensive quality control measures begins with sample preparation standardization, where particle size distribution must be controlled within ±5 μm and sample mass maintained at 50 ± 0.5 mg to minimize thermal gradient effects. Variations in these parameters have been shown to significantly alter peak temperatures and reaction kinetics in both minerals.
Reference materials must be selected with consideration for the thermal properties of rhodochrosite and hematite. For rhodochrosite analysis, calcined alumina serves as an optimal reference due to its thermal stability through the critical decomposition range (350-650°C), while for hematite studies, platinum oxide provides better baseline stability at higher temperatures (600-900°C) where phase transitions occur.
Calibration procedures require multi-point temperature verification using at least five standard materials spanning 100-1000°C, with indium, tin, zinc, aluminum, and silver being the preferred calibrants. Quarterly recalibration is necessary to account for thermocouple drift, with verification runs conducted weekly using a rhodochrosite standard (99.99% purity) to detect any instrument deviation.
Atmosphere control represents a critical quality parameter, as rhodochrosite decomposition kinetics are particularly sensitive to oxygen partial pressure. Standardized protocols should specify nitrogen flow rates of 50 ± 2 mL/min for inert conditions or precisely controlled oxygen concentrations (21.0 ± 0.5%) for oxidative studies. Humidity must be maintained below 2% relative humidity to prevent secondary hydration reactions.
Data processing standardization requires baseline correction using polynomial fitting methods rather than linear approximations, which inadequately account for the complex baseline shifts observed during rhodochrosite decomposition. Peak integration boundaries must be defined at 5% of peak height to ensure consistent enthalpy calculations across different instrument platforms.
Interlaboratory validation studies have demonstrated that adherence to these protocols can reduce measurement uncertainty from ±15% to ±3% for decomposition temperatures and from ±22% to ±7% for reaction enthalpies. Implementation of statistical process control charts for monitoring key parameters such as peak temperature, onset temperature, and enthalpy provides early detection of instrumental or procedural drift.
Documentation requirements include comprehensive metadata recording sample history, preparation methods, and environmental conditions during analysis, enabling meaningful comparison of results across different research groups and facilitating the development of shared databases for mineral thermal properties.
Reference materials must be selected with consideration for the thermal properties of rhodochrosite and hematite. For rhodochrosite analysis, calcined alumina serves as an optimal reference due to its thermal stability through the critical decomposition range (350-650°C), while for hematite studies, platinum oxide provides better baseline stability at higher temperatures (600-900°C) where phase transitions occur.
Calibration procedures require multi-point temperature verification using at least five standard materials spanning 100-1000°C, with indium, tin, zinc, aluminum, and silver being the preferred calibrants. Quarterly recalibration is necessary to account for thermocouple drift, with verification runs conducted weekly using a rhodochrosite standard (99.99% purity) to detect any instrument deviation.
Atmosphere control represents a critical quality parameter, as rhodochrosite decomposition kinetics are particularly sensitive to oxygen partial pressure. Standardized protocols should specify nitrogen flow rates of 50 ± 2 mL/min for inert conditions or precisely controlled oxygen concentrations (21.0 ± 0.5%) for oxidative studies. Humidity must be maintained below 2% relative humidity to prevent secondary hydration reactions.
Data processing standardization requires baseline correction using polynomial fitting methods rather than linear approximations, which inadequately account for the complex baseline shifts observed during rhodochrosite decomposition. Peak integration boundaries must be defined at 5% of peak height to ensure consistent enthalpy calculations across different instrument platforms.
Interlaboratory validation studies have demonstrated that adherence to these protocols can reduce measurement uncertainty from ±15% to ±3% for decomposition temperatures and from ±22% to ±7% for reaction enthalpies. Implementation of statistical process control charts for monitoring key parameters such as peak temperature, onset temperature, and enthalpy provides early detection of instrumental or procedural drift.
Documentation requirements include comprehensive metadata recording sample history, preparation methods, and environmental conditions during analysis, enabling meaningful comparison of results across different research groups and facilitating the development of shared databases for mineral thermal properties.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!