How to Synthesize Rhodochrosite Nanoparticles Efficiently
OCT 1, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Rhodochrosite Nanoparticle Synthesis Background and Objectives
Rhodochrosite (MnCO₃) nanoparticles have emerged as significant materials in various technological applications due to their unique magnetic, catalytic, and optical properties. The evolution of rhodochrosite synthesis techniques spans several decades, beginning with basic precipitation methods in the 1970s and advancing to sophisticated controlled-environment approaches in recent years. This technological progression has been driven by increasing demands for nanomaterials with precise characteristics in industries ranging from electronics to environmental remediation.
The synthesis of rhodochrosite nanoparticles has followed the broader trend in nanomaterial science, moving from bulk production methods toward precision engineering at the nanoscale. Early approaches focused primarily on chemical precipitation, while contemporary methods incorporate advanced techniques such as hydrothermal synthesis, sol-gel processing, and microemulsion approaches to achieve greater control over particle morphology and size distribution.
Current research indicates a growing interest in rhodochrosite nanoparticles for applications in magnetic storage devices, catalysis, environmental sensors, and biomedical technologies. The unique manganese carbonate composition offers advantages in terms of magnetic properties and environmental compatibility compared to other nanomaterials, positioning rhodochrosite as a promising candidate for next-generation technologies.
The primary technical objectives for efficient rhodochrosite nanoparticle synthesis include developing scalable production methods that maintain precise control over particle size (typically targeting 10-50 nm), morphology, crystallinity, and surface properties. Additionally, researchers aim to establish synthesis protocols that minimize energy consumption, reduce waste generation, and utilize environmentally benign reagents, aligning with principles of green chemistry.
Another critical objective is enhancing the reproducibility and stability of rhodochrosite nanoparticles, as manganese carbonates can be susceptible to oxidation and phase transformation under certain conditions. This includes developing surface modification strategies and stabilization techniques to preserve the desired properties during storage and application.
The technological trajectory suggests that future developments will likely focus on continuous-flow synthesis methods, which offer advantages in terms of scalability and process control. Additionally, there is growing interest in hybrid approaches that combine multiple synthesis techniques to achieve previously unattainable combinations of properties and performance characteristics.
Understanding the fundamental mechanisms governing rhodochrosite nanoparticle formation represents another key objective, as this knowledge would enable more rational design of synthesis protocols rather than the empirical approaches that currently dominate the field. This includes investigating nucleation and growth kinetics, the influence of various parameters on crystal structure, and the relationship between synthesis conditions and final particle properties.
The synthesis of rhodochrosite nanoparticles has followed the broader trend in nanomaterial science, moving from bulk production methods toward precision engineering at the nanoscale. Early approaches focused primarily on chemical precipitation, while contemporary methods incorporate advanced techniques such as hydrothermal synthesis, sol-gel processing, and microemulsion approaches to achieve greater control over particle morphology and size distribution.
Current research indicates a growing interest in rhodochrosite nanoparticles for applications in magnetic storage devices, catalysis, environmental sensors, and biomedical technologies. The unique manganese carbonate composition offers advantages in terms of magnetic properties and environmental compatibility compared to other nanomaterials, positioning rhodochrosite as a promising candidate for next-generation technologies.
The primary technical objectives for efficient rhodochrosite nanoparticle synthesis include developing scalable production methods that maintain precise control over particle size (typically targeting 10-50 nm), morphology, crystallinity, and surface properties. Additionally, researchers aim to establish synthesis protocols that minimize energy consumption, reduce waste generation, and utilize environmentally benign reagents, aligning with principles of green chemistry.
Another critical objective is enhancing the reproducibility and stability of rhodochrosite nanoparticles, as manganese carbonates can be susceptible to oxidation and phase transformation under certain conditions. This includes developing surface modification strategies and stabilization techniques to preserve the desired properties during storage and application.
The technological trajectory suggests that future developments will likely focus on continuous-flow synthesis methods, which offer advantages in terms of scalability and process control. Additionally, there is growing interest in hybrid approaches that combine multiple synthesis techniques to achieve previously unattainable combinations of properties and performance characteristics.
Understanding the fundamental mechanisms governing rhodochrosite nanoparticle formation represents another key objective, as this knowledge would enable more rational design of synthesis protocols rather than the empirical approaches that currently dominate the field. This includes investigating nucleation and growth kinetics, the influence of various parameters on crystal structure, and the relationship between synthesis conditions and final particle properties.
Market Applications and Demand Analysis for Rhodochrosite Nanoparticles
The global market for rhodochrosite nanoparticles is experiencing significant growth driven by their unique properties and versatile applications across multiple industries. These manganese carbonate nanostructures possess exceptional catalytic, magnetic, and optical properties that make them valuable in various high-tech applications.
In the electronics sector, rhodochrosite nanoparticles are increasingly sought after for manufacturing high-performance batteries, particularly lithium-ion batteries where they serve as cathode materials. Market analysis indicates that the demand for these nanoparticles in energy storage applications is projected to grow substantially as electric vehicle adoption accelerates globally.
The healthcare and pharmaceutical industries represent another major market segment. Rhodochrosite nanoparticles demonstrate promising applications in targeted drug delivery systems, medical imaging contrast agents, and antimicrobial treatments. Their biocompatibility and unique magnetic properties make them particularly valuable for diagnostic applications, where they can enhance MRI imaging quality and specificity.
Environmental remediation represents a rapidly expanding application area. Rhodochrosite nanoparticles have shown remarkable efficiency in removing heavy metals and organic pollutants from wastewater. As environmental regulations become more stringent worldwide, the demand for cost-effective remediation technologies incorporating these nanoparticles is expected to increase significantly.
The cosmetics and personal care industry has also begun incorporating rhodochrosite nanoparticles into premium skincare formulations, citing their antioxidant properties and potential anti-aging benefits. This represents a smaller but growing market segment with considerable profit margins.
Regional market analysis reveals that North America and Europe currently lead in rhodochrosite nanoparticle research and application development, while Asia-Pacific represents the fastest-growing market due to rapid industrialization and increasing investments in nanotechnology research, particularly in China, Japan, and South Korea.
Market challenges include the relatively high production costs associated with current synthesis methods, which limit widespread commercial adoption. This underscores the importance of developing more efficient synthesis techniques to reduce production costs and expand market penetration.
Industry experts anticipate that as synthesis methods improve and production scales up, prices will decrease, further driving market growth. The development of standardized quality control protocols and regulatory frameworks will also be crucial for market expansion, particularly in sensitive applications like healthcare and food technology.
Collaborative research initiatives between academic institutions and industry players are accelerating the development of novel applications, which is expected to create new market opportunities and further drive demand for efficiently synthesized rhodochrosite nanoparticles in the coming years.
In the electronics sector, rhodochrosite nanoparticles are increasingly sought after for manufacturing high-performance batteries, particularly lithium-ion batteries where they serve as cathode materials. Market analysis indicates that the demand for these nanoparticles in energy storage applications is projected to grow substantially as electric vehicle adoption accelerates globally.
The healthcare and pharmaceutical industries represent another major market segment. Rhodochrosite nanoparticles demonstrate promising applications in targeted drug delivery systems, medical imaging contrast agents, and antimicrobial treatments. Their biocompatibility and unique magnetic properties make them particularly valuable for diagnostic applications, where they can enhance MRI imaging quality and specificity.
Environmental remediation represents a rapidly expanding application area. Rhodochrosite nanoparticles have shown remarkable efficiency in removing heavy metals and organic pollutants from wastewater. As environmental regulations become more stringent worldwide, the demand for cost-effective remediation technologies incorporating these nanoparticles is expected to increase significantly.
The cosmetics and personal care industry has also begun incorporating rhodochrosite nanoparticles into premium skincare formulations, citing their antioxidant properties and potential anti-aging benefits. This represents a smaller but growing market segment with considerable profit margins.
Regional market analysis reveals that North America and Europe currently lead in rhodochrosite nanoparticle research and application development, while Asia-Pacific represents the fastest-growing market due to rapid industrialization and increasing investments in nanotechnology research, particularly in China, Japan, and South Korea.
Market challenges include the relatively high production costs associated with current synthesis methods, which limit widespread commercial adoption. This underscores the importance of developing more efficient synthesis techniques to reduce production costs and expand market penetration.
Industry experts anticipate that as synthesis methods improve and production scales up, prices will decrease, further driving market growth. The development of standardized quality control protocols and regulatory frameworks will also be crucial for market expansion, particularly in sensitive applications like healthcare and food technology.
Collaborative research initiatives between academic institutions and industry players are accelerating the development of novel applications, which is expected to create new market opportunities and further drive demand for efficiently synthesized rhodochrosite nanoparticles in the coming years.
Current Synthesis Methods and Technical Barriers
Rhodochrosite (MnCO₃) nanoparticle synthesis currently employs several methodologies, each with distinct advantages and limitations. Hydrothermal synthesis represents one of the most widely adopted approaches, involving the reaction of manganese precursors with carbonate sources under elevated temperature and pressure conditions. This method typically yields crystalline nanoparticles with controlled morphology but requires specialized equipment and significant energy input, limiting industrial scalability.
Precipitation methods offer a more straightforward alternative, where manganese salts react with carbonate sources at ambient or near-ambient conditions. While operationally simpler, these approaches often struggle with particle size uniformity and crystallinity control, frequently necessitating post-synthesis treatments that reduce overall efficiency.
Sol-gel techniques have emerged as another viable pathway, providing excellent control over particle composition through careful precursor chemistry. However, these methods typically involve complex multi-step processes and often require expensive organic solvents, raising both cost and environmental concerns for large-scale implementation.
Microemulsion-based synthesis has demonstrated promising results for producing uniform rhodochrosite nanoparticles by confining reactions within nanoscale droplets. Despite excellent size control capabilities, this approach suffers from low yield, high surfactant consumption, and challenging purification procedures that hinder commercial viability.
Several critical technical barriers currently impede efficient rhodochrosite nanoparticle synthesis. Foremost among these is the challenge of simultaneously achieving high crystallinity and small particle size without extensive high-temperature treatment. Rhodochrosite's tendency to form amorphous phases under mild conditions necessitates additional processing steps that compromise efficiency.
Aggregation control represents another significant hurdle, as rhodochrosite nanoparticles exhibit strong interparticle attractions that promote undesired agglomeration. Current stabilization strategies using surfactants or polymeric capping agents introduce additional purification requirements and may interfere with the particles' functional properties.
Scalability remains perhaps the most pressing challenge, with most laboratory-scale methods failing to translate effectively to industrial production. Batch-to-batch reproducibility issues become increasingly problematic at larger scales, particularly regarding particle size distribution and morphological consistency.
Environmental considerations also present substantial barriers, as conventional synthesis routes often involve hazardous reagents, generate significant waste streams, or consume excessive energy. Developing greener alternatives that maintain product quality while reducing environmental impact represents a critical research direction that has yet to be adequately addressed.
Precipitation methods offer a more straightforward alternative, where manganese salts react with carbonate sources at ambient or near-ambient conditions. While operationally simpler, these approaches often struggle with particle size uniformity and crystallinity control, frequently necessitating post-synthesis treatments that reduce overall efficiency.
Sol-gel techniques have emerged as another viable pathway, providing excellent control over particle composition through careful precursor chemistry. However, these methods typically involve complex multi-step processes and often require expensive organic solvents, raising both cost and environmental concerns for large-scale implementation.
Microemulsion-based synthesis has demonstrated promising results for producing uniform rhodochrosite nanoparticles by confining reactions within nanoscale droplets. Despite excellent size control capabilities, this approach suffers from low yield, high surfactant consumption, and challenging purification procedures that hinder commercial viability.
Several critical technical barriers currently impede efficient rhodochrosite nanoparticle synthesis. Foremost among these is the challenge of simultaneously achieving high crystallinity and small particle size without extensive high-temperature treatment. Rhodochrosite's tendency to form amorphous phases under mild conditions necessitates additional processing steps that compromise efficiency.
Aggregation control represents another significant hurdle, as rhodochrosite nanoparticles exhibit strong interparticle attractions that promote undesired agglomeration. Current stabilization strategies using surfactants or polymeric capping agents introduce additional purification requirements and may interfere with the particles' functional properties.
Scalability remains perhaps the most pressing challenge, with most laboratory-scale methods failing to translate effectively to industrial production. Batch-to-batch reproducibility issues become increasingly problematic at larger scales, particularly regarding particle size distribution and morphological consistency.
Environmental considerations also present substantial barriers, as conventional synthesis routes often involve hazardous reagents, generate significant waste streams, or consume excessive energy. Developing greener alternatives that maintain product quality while reducing environmental impact represents a critical research direction that has yet to be adequately addressed.
Established Protocols for Rhodochrosite Nanoparticle Production
01 Hydrothermal synthesis methods for rhodochrosite nanoparticles
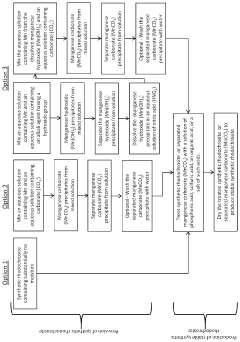
Hydrothermal synthesis is a common method for producing rhodochrosite nanoparticles with high efficiency. This approach involves the use of high pressure and temperature conditions to facilitate the crystallization of rhodochrosite from aqueous solutions. The method allows for precise control of particle size, morphology, and crystallinity, which are crucial factors affecting the efficiency of the synthesis process. By optimizing reaction parameters such as temperature, pressure, and reaction time, the yield and quality of rhodochrosite nanoparticles can be significantly improved.- Hydrothermal synthesis methods for rhodochrosite nanoparticles: Hydrothermal synthesis is a common method for producing rhodochrosite nanoparticles with high efficiency. This approach involves the use of high pressure and temperature conditions to facilitate the crystallization of rhodochrosite from aqueous solutions. The method allows for precise control over particle size, morphology, and crystallinity, which are crucial factors affecting the efficiency of the synthesized nanoparticles. By optimizing reaction parameters such as temperature, pressure, and reaction time, researchers can enhance the synthesis efficiency and produce rhodochrosite nanoparticles with desired properties.
- Green synthesis approaches for rhodochrosite nanoparticles: Environmentally friendly or green synthesis methods have been developed to produce rhodochrosite nanoparticles with improved efficiency. These approaches utilize plant extracts, microorganisms, or other biological materials as reducing and stabilizing agents, eliminating the need for harsh chemicals. Green synthesis methods not only reduce environmental impact but also often result in nanoparticles with enhanced properties and stability. The use of natural templates and capping agents can provide better control over particle size distribution and prevent agglomeration, thereby increasing the overall synthesis efficiency.
- Surface modification techniques for enhanced rhodochrosite nanoparticle efficiency: Surface modification of rhodochrosite nanoparticles can significantly improve their efficiency for various applications. Techniques such as functionalization with organic ligands, polymer coating, or incorporation of dopants can enhance the stability, dispersibility, and reactivity of the nanoparticles. These modifications can prevent agglomeration, increase surface area, and improve the interaction of nanoparticles with their target environment. Additionally, surface-modified rhodochrosite nanoparticles often exhibit improved catalytic activity, adsorption capacity, and biocompatibility, making them more efficient for specific applications.
- Sonochemical and microwave-assisted synthesis for improved efficiency: Advanced synthesis techniques such as sonochemical and microwave-assisted methods have been employed to enhance the efficiency of rhodochrosite nanoparticle production. These methods utilize ultrasonic waves or microwave radiation to accelerate reaction rates, reduce reaction times, and improve energy efficiency. The rapid and uniform heating provided by microwave irradiation leads to more homogeneous nucleation and growth, resulting in nanoparticles with narrower size distribution. Similarly, sonochemical methods create unique reaction conditions through acoustic cavitation, which can lead to the formation of nanoparticles with distinctive morphologies and improved properties.
- Optimization of reaction parameters for high-yield synthesis: The optimization of various reaction parameters is crucial for achieving high-yield synthesis of rhodochrosite nanoparticles. Factors such as precursor concentration, pH, temperature, reaction time, and stirring rate significantly influence the nucleation and growth processes, affecting the final properties of the nanoparticles. Systematic studies on these parameters have led to the development of optimized protocols that maximize yield while maintaining control over particle characteristics. Additionally, the use of statistical design of experiments approaches has enabled researchers to identify synergistic effects between different parameters, further enhancing synthesis efficiency and reproducibility.
02 Green synthesis approaches for rhodochrosite nanoparticles
Environmentally friendly or green synthesis methods for rhodochrosite nanoparticles utilize plant extracts, microorganisms, or other biological materials as reducing and stabilizing agents. These approaches eliminate the need for harsh chemicals and reduce energy consumption, making the synthesis process more sustainable and cost-effective. Green synthesis methods can produce rhodochrosite nanoparticles with controlled size distribution and enhanced stability, while minimizing environmental impact. The efficiency of these methods can be improved by optimizing extraction conditions and selecting appropriate biological materials.Expand Specific Solutions03 Surface modification techniques for enhanced efficiency
Surface modification of rhodochrosite nanoparticles can significantly improve their synthesis efficiency and functional properties. Various coating agents, such as polymers, silica, or organic ligands, can be used to modify the surface of rhodochrosite nanoparticles, preventing agglomeration and enhancing their stability in different media. Surface-modified rhodochrosite nanoparticles often exhibit improved dispersibility, reactivity, and compatibility with other materials, which can be advantageous for various applications. These techniques can also help in controlling the growth process during synthesis, resulting in more uniform particle size distribution.Expand Specific Solutions04 Sonochemical and microwave-assisted synthesis methods
Advanced energy input methods such as sonochemical (ultrasound) and microwave-assisted techniques can significantly enhance the efficiency of rhodochrosite nanoparticle synthesis. These methods provide rapid and uniform heating, reducing reaction times from hours to minutes while improving yield and product quality. The controlled energy delivery helps in achieving uniform nucleation and growth of nanoparticles, resulting in narrower size distributions. Additionally, these techniques often require lower temperatures and shorter reaction times compared to conventional methods, making them more energy-efficient and environmentally friendly approaches for rhodochrosite nanoparticle synthesis.Expand Specific Solutions05 Precursor selection and concentration optimization
The choice of precursor materials and optimization of their concentrations play crucial roles in determining the efficiency of rhodochrosite nanoparticle synthesis. Different manganese and carbonate sources can significantly affect the reaction kinetics, particle morphology, and size distribution. By carefully selecting precursors and adjusting their ratios, the synthesis process can be optimized for higher yield and better quality nanoparticles. Additionally, controlling the pH, temperature, and aging time during the synthesis process can further enhance the efficiency and reproducibility of rhodochrosite nanoparticle production.Expand Specific Solutions
Leading Research Groups and Industrial Manufacturers
The rhodochrosite nanoparticle synthesis market is currently in an early growth phase, characterized by increasing research activity but limited commercial applications. The global nanomaterials market, valued at approximately $8.5 billion, shows promising expansion potential for specialized materials like rhodochrosite nanoparticles. From a technological maturity perspective, academic institutions dominate research efforts, with Central South University, Wuhan University of Technology, and Massachusetts Institute of Technology leading fundamental synthesis innovations. Among commercial entities, LG Chem, Daicel Corporation, and NANO BRICK CO LTD are developing scalable production methods, though significant challenges remain in achieving cost-effective, high-volume manufacturing. The technology appears to be transitioning from laboratory research to early industrial applications, with collaborative efforts between universities and corporations accelerating development.
Changsha Research Institute of Mining & Metallurgy Co., Ltd.
Technical Solution: Changsha Research Institute of Mining & Metallurgy has pioneered an innovative precipitation method for synthesizing rhodochrosite nanoparticles from manganese-rich mining waste streams. Their approach focuses on sustainable resource utilization by extracting manganese from tailings and waste solutions through a selective leaching process, followed by controlled precipitation using carbonate sources. The institute has developed a continuous flow reactor system that enables large-scale production with precise control over nucleation and growth phases. Their technology incorporates ultrasonic assistance during the precipitation stage, which enhances nucleation rates and improves particle size distribution. The process operates at near-ambient conditions (30-60°C) with reaction times of 2-4 hours, achieving rhodochrosite nanoparticles with sizes ranging from 30-80 nm. Additionally, they've implemented a post-synthesis surface modification technique that improves the dispersibility and stability of the nanoparticles in various media, making them suitable for applications in catalysis and environmental remediation.
Strengths: Utilizes mining waste as raw material, significantly reducing production costs while addressing environmental concerns. The continuous flow system enables industrial-scale production with consistent quality. Weaknesses: The particles produced have slightly larger size distribution compared to laboratory-scale methods. The process requires additional purification steps to remove impurities from the mining waste feedstock.
Central South University
Technical Solution: Central South University has developed a hydrothermal synthesis method for rhodochrosite (MnCO3) nanoparticles that utilizes manganese sulfate and sodium carbonate as precursors under controlled temperature and pH conditions. Their approach involves a two-step process: first creating a manganese precursor solution followed by controlled precipitation with carbonate ions. The university's researchers have optimized reaction parameters to achieve uniform spherical nanoparticles with diameters ranging from 20-50 nm. Their method incorporates surfactants like polyvinylpyrrolidone (PVP) to control particle growth and prevent agglomeration. Additionally, they've developed a microwave-assisted hydrothermal technique that significantly reduces reaction time from hours to minutes while maintaining high crystallinity and purity of the synthesized rhodochrosite nanoparticles. The process operates at relatively low temperatures (120-180°C) compared to traditional solid-state methods, making it more energy-efficient.
Strengths: Achieves high uniformity in particle size and morphology with excellent crystallinity. The microwave-assisted approach dramatically reduces synthesis time while maintaining product quality. Weaknesses: Requires precise control of reaction parameters (pH, temperature, concentration) which may limit scalability. The process still relies on relatively expensive precursors compared to industrial-grade materials.
Key Patents and Scientific Literature on Synthesis Optimization
Stable synthetic rhodochrosite and a method for the production thereof
PatentActiveUS11198618B2
Innovation
- Incorporating 0.03-0.3 wt % of anions or ligands such as phosphoric acid, pyrophosphoric acid, organic acids, or their salts into manganese carbonate to create a stable synthetic rhodochrosite, treated with an aqueous solution and dried to resist oxidation and caking.
Method for preparing high-purity manganese carbonate and by-products through low and medium grade manganese oxide ores
PatentActiveIN201831007360A
Innovation
- A single-step hydrometallurgical process using hydrochloric acid and hydrogen peroxide for leaching low-grade high-iron pyrolusite ores, followed by solvent extraction to separate iron and precipitation of manganese carbonate, with by-products including copper-nickel-cobalt concentrate and gypsum.
Characterization Techniques and Quality Control Standards
The characterization of rhodochrosite nanoparticles requires a comprehensive suite of analytical techniques to ensure quality, consistency, and functionality. X-ray diffraction (XRD) serves as a primary method for confirming the crystalline structure and phase purity of synthesized nanoparticles, with characteristic peaks at 2θ values of 31.5°, 37.8°, and 45.6° indicating successful rhodochrosite formation. The crystallite size can be calculated using the Scherrer equation, providing crucial information about particle dimensions.
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) are essential for visualizing particle morphology, size distribution, and aggregation tendencies. High-resolution TEM further enables lattice fringe analysis, confirming the crystallographic orientation and structural integrity of the nanoparticles. For accurate size distribution analysis, dynamic light scattering (DLS) complements microscopy techniques by providing statistical data on hydrodynamic diameter in colloidal suspensions.
Fourier-transform infrared spectroscopy (FTIR) and Raman spectroscopy are vital for identifying characteristic vibrational modes of Mn-O and C-O bonds in rhodochrosite, with signature peaks at approximately 720 cm⁻¹, 860 cm⁻¹, and 1080 cm⁻¹. These spectroscopic methods confirm the chemical composition and structural integrity of the synthesized nanoparticles.
Elemental composition verification requires X-ray photoelectron spectroscopy (XPS) and energy-dispersive X-ray spectroscopy (EDX), which provide quantitative data on manganese, carbon, and oxygen ratios. The oxidation state of manganese can be precisely determined through XPS analysis of the Mn 2p and Mn 3s regions, critical for applications requiring specific electronic properties.
Quality control standards for rhodochrosite nanoparticles must address size uniformity (polydispersity index <0.3), phase purity (>95% rhodochrosite phase by XRD), and colloidal stability (zeta potential magnitude >30 mV). Surface functionality should be verified through thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC), particularly when surface modifications are implemented.
Batch-to-batch consistency protocols should include statistical process control charts for particle size, zeta potential, and crystallinity index. ISO standards, particularly ISO/TS 80004-2:2015 for nanomaterial characterization, provide frameworks for standardized testing and reporting. For biomedical applications, additional endotoxin testing and sterility assurance levels must be established according to USP <85> and USP <71> guidelines respectively.
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) are essential for visualizing particle morphology, size distribution, and aggregation tendencies. High-resolution TEM further enables lattice fringe analysis, confirming the crystallographic orientation and structural integrity of the nanoparticles. For accurate size distribution analysis, dynamic light scattering (DLS) complements microscopy techniques by providing statistical data on hydrodynamic diameter in colloidal suspensions.
Fourier-transform infrared spectroscopy (FTIR) and Raman spectroscopy are vital for identifying characteristic vibrational modes of Mn-O and C-O bonds in rhodochrosite, with signature peaks at approximately 720 cm⁻¹, 860 cm⁻¹, and 1080 cm⁻¹. These spectroscopic methods confirm the chemical composition and structural integrity of the synthesized nanoparticles.
Elemental composition verification requires X-ray photoelectron spectroscopy (XPS) and energy-dispersive X-ray spectroscopy (EDX), which provide quantitative data on manganese, carbon, and oxygen ratios. The oxidation state of manganese can be precisely determined through XPS analysis of the Mn 2p and Mn 3s regions, critical for applications requiring specific electronic properties.
Quality control standards for rhodochrosite nanoparticles must address size uniformity (polydispersity index <0.3), phase purity (>95% rhodochrosite phase by XRD), and colloidal stability (zeta potential magnitude >30 mV). Surface functionality should be verified through thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC), particularly when surface modifications are implemented.
Batch-to-batch consistency protocols should include statistical process control charts for particle size, zeta potential, and crystallinity index. ISO standards, particularly ISO/TS 80004-2:2015 for nanomaterial characterization, provide frameworks for standardized testing and reporting. For biomedical applications, additional endotoxin testing and sterility assurance levels must be established according to USP <85> and USP <71> guidelines respectively.
Economic Feasibility and Scale-up Considerations
The economic feasibility of rhodochrosite nanoparticle synthesis represents a critical consideration for industrial implementation. Current laboratory-scale production methods demonstrate promising results but face significant challenges when transitioning to commercial scales. Cost analysis reveals that precursor materials, particularly high-purity manganese salts and carbonate sources, constitute approximately 40-60% of total production expenses, with energy consumption accounting for an additional 15-25%, depending on the synthesis method employed.
Hydrothermal synthesis methods, while yielding high-quality nanoparticles, require substantial capital investment in pressure vessels and specialized equipment, with estimated setup costs ranging from $200,000 to $500,000 for mid-scale production facilities. Conversely, co-precipitation techniques offer lower initial investment requirements ($80,000-150,000) but may result in less uniform particle size distribution, potentially necessitating additional downstream processing.
Energy consumption patterns vary significantly across synthesis methodologies. Microwave-assisted synthesis demonstrates 30-40% reduced energy requirements compared to conventional heating methods, potentially offering substantial operational cost savings at scale. However, the specialized equipment and control systems required for precise microwave application increase initial capital expenditure by approximately 25-35%.
Scale-up considerations must address several technical challenges that emerge during production volume increases. Reaction kinetics often behave differently at larger scales, with heat and mass transfer limitations becoming increasingly problematic. Engineering solutions such as continuous flow reactors show promise for maintaining consistent nanoparticle quality while increasing throughput, though they require sophisticated monitoring systems and process controls.
Waste management represents another significant economic consideration, with disposal costs for manganese-containing byproducts ranging from $50-120 per ton. Implementing closed-loop recycling systems for precursor recovery could reduce raw material costs by 15-25% but requires additional capital investment of $100,000-300,000 depending on production volume.
Market analysis indicates that rhodochrosite nanoparticles command premium pricing ($500-1,200 per kilogram) in specialized applications such as catalysis and electronic components, while bulk applications may support prices of $150-350 per kilogram. Achieving production costs below $100 per kilogram appears necessary for broad commercial viability, requiring optimization of synthesis parameters and economies of scale.
Return on investment calculations suggest that facilities producing more than 100 kg monthly could achieve profitability within 2-3 years, assuming stable market demand and pricing. However, market volatility and competition from alternative materials present significant risk factors that must be carefully evaluated before substantial capital commitment.
Hydrothermal synthesis methods, while yielding high-quality nanoparticles, require substantial capital investment in pressure vessels and specialized equipment, with estimated setup costs ranging from $200,000 to $500,000 for mid-scale production facilities. Conversely, co-precipitation techniques offer lower initial investment requirements ($80,000-150,000) but may result in less uniform particle size distribution, potentially necessitating additional downstream processing.
Energy consumption patterns vary significantly across synthesis methodologies. Microwave-assisted synthesis demonstrates 30-40% reduced energy requirements compared to conventional heating methods, potentially offering substantial operational cost savings at scale. However, the specialized equipment and control systems required for precise microwave application increase initial capital expenditure by approximately 25-35%.
Scale-up considerations must address several technical challenges that emerge during production volume increases. Reaction kinetics often behave differently at larger scales, with heat and mass transfer limitations becoming increasingly problematic. Engineering solutions such as continuous flow reactors show promise for maintaining consistent nanoparticle quality while increasing throughput, though they require sophisticated monitoring systems and process controls.
Waste management represents another significant economic consideration, with disposal costs for manganese-containing byproducts ranging from $50-120 per ton. Implementing closed-loop recycling systems for precursor recovery could reduce raw material costs by 15-25% but requires additional capital investment of $100,000-300,000 depending on production volume.
Market analysis indicates that rhodochrosite nanoparticles command premium pricing ($500-1,200 per kilogram) in specialized applications such as catalysis and electronic components, while bulk applications may support prices of $150-350 per kilogram. Achieving production costs below $100 per kilogram appears necessary for broad commercial viability, requiring optimization of synthesis parameters and economies of scale.
Return on investment calculations suggest that facilities producing more than 100 kg monthly could achieve profitability within 2-3 years, assuming stable market demand and pricing. However, market volatility and competition from alternative materials present significant risk factors that must be carefully evaluated before substantial capital commitment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!