How to Analyze Rhodochrosite Formation in Sedimentary Rocks
OCT 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Rhodochrosite Formation Background and Research Objectives
Rhodochrosite (MnCO₃) represents a significant manganese carbonate mineral that forms under specific geological conditions in sedimentary environments. The study of rhodochrosite formation has evolved considerably over the past century, from basic descriptive mineralogy to sophisticated geochemical analysis incorporating isotope studies and advanced imaging techniques. This mineral's distinctive pink to red coloration makes it both scientifically valuable and commercially desirable as a semi-precious gemstone.
The formation of rhodochrosite in sedimentary rocks occurs primarily through diagenetic processes in manganese-rich environments under specific redox conditions. Historical research has established that rhodochrosite typically forms in environments where manganese reduction occurs in the presence of sufficient carbonate ions, often in association with organic matter degradation. Recent technological advancements have significantly enhanced our ability to analyze the precise mechanisms and environmental conditions governing rhodochrosite precipitation.
Current research trends indicate growing interest in rhodochrosite as both an economic resource and as a paleoenvironmental indicator. The mineral's composition and structural characteristics can provide valuable insights into ancient depositional environments, including redox conditions, microbial activity, and diagenetic processes. Additionally, the increasing industrial demand for manganese has heightened interest in understanding rhodochrosite formation as part of broader manganese ore genesis studies.
The primary objective of this technical research is to comprehensively evaluate current analytical methodologies for studying rhodochrosite formation in sedimentary rocks. This includes assessment of traditional petrographic techniques, modern spectroscopic methods, isotope geochemistry approaches, and advanced imaging technologies. By synthesizing these diverse analytical approaches, we aim to develop an integrated framework for rhodochrosite formation analysis that can be applied across various geological settings.
Secondary objectives include identifying key environmental parameters controlling rhodochrosite precipitation, evaluating the role of microbial mediation in its formation, and establishing reliable indicators for distinguishing primary from secondary rhodochrosite in ancient sedimentary sequences. Furthermore, this research seeks to explore the potential of rhodochrosite as a paleoenvironmental proxy, particularly for reconstructing ancient marine and lacustrine conditions where traditional indicators may be limited.
The technological evolution in analytical instrumentation, particularly in high-resolution microscopy, synchrotron-based techniques, and in-situ isotope analysis, has opened new avenues for rhodochrosite research that were previously inaccessible. These advancements provide unprecedented opportunities to resolve longstanding questions regarding the precise mechanisms of rhodochrosite formation and their implications for both economic geology and Earth history.
The formation of rhodochrosite in sedimentary rocks occurs primarily through diagenetic processes in manganese-rich environments under specific redox conditions. Historical research has established that rhodochrosite typically forms in environments where manganese reduction occurs in the presence of sufficient carbonate ions, often in association with organic matter degradation. Recent technological advancements have significantly enhanced our ability to analyze the precise mechanisms and environmental conditions governing rhodochrosite precipitation.
Current research trends indicate growing interest in rhodochrosite as both an economic resource and as a paleoenvironmental indicator. The mineral's composition and structural characteristics can provide valuable insights into ancient depositional environments, including redox conditions, microbial activity, and diagenetic processes. Additionally, the increasing industrial demand for manganese has heightened interest in understanding rhodochrosite formation as part of broader manganese ore genesis studies.
The primary objective of this technical research is to comprehensively evaluate current analytical methodologies for studying rhodochrosite formation in sedimentary rocks. This includes assessment of traditional petrographic techniques, modern spectroscopic methods, isotope geochemistry approaches, and advanced imaging technologies. By synthesizing these diverse analytical approaches, we aim to develop an integrated framework for rhodochrosite formation analysis that can be applied across various geological settings.
Secondary objectives include identifying key environmental parameters controlling rhodochrosite precipitation, evaluating the role of microbial mediation in its formation, and establishing reliable indicators for distinguishing primary from secondary rhodochrosite in ancient sedimentary sequences. Furthermore, this research seeks to explore the potential of rhodochrosite as a paleoenvironmental proxy, particularly for reconstructing ancient marine and lacustrine conditions where traditional indicators may be limited.
The technological evolution in analytical instrumentation, particularly in high-resolution microscopy, synchrotron-based techniques, and in-situ isotope analysis, has opened new avenues for rhodochrosite research that were previously inaccessible. These advancements provide unprecedented opportunities to resolve longstanding questions regarding the precise mechanisms of rhodochrosite formation and their implications for both economic geology and Earth history.
Market Applications and Demand for Rhodochrosite Analysis
The global market for rhodochrosite analysis in sedimentary rocks has experienced significant growth in recent years, primarily driven by expanding applications in mining, geology, and materials science sectors. The mineral's distinctive pink to red coloration and manganese content make it valuable both as a gemstone and as an industrial resource, creating diverse market demands for analytical techniques.
In the mining industry, accurate rhodochrosite formation analysis has become essential for exploration companies seeking to identify viable manganese deposits. The global manganese market, valued at approximately $20 billion annually, relies heavily on precise mineral identification and characterization techniques. Companies investing in exploration activities require sophisticated analytical methods to reduce financial risk and optimize resource extraction strategies.
The gemstone market represents another significant demand driver, with rhodochrosite specimens commanding premium prices in collector and jewelry markets. High-quality rhodochrosite from notable locations such as the Sweet Home Mine in Colorado can fetch substantial prices, creating demand for authentication and quality assessment techniques among gemologists and mineral dealers.
Environmental monitoring and remediation sectors have emerged as growth areas for rhodochrosite analysis. As manganese contamination becomes an increasing concern in water systems, the ability to understand rhodochrosite formation and dissolution processes has direct applications in environmental management and remediation technology development.
Academic and research institutions constitute a stable market segment, with ongoing research into sedimentary processes, paleoenvironmental reconstruction, and geochemical cycling creating consistent demand for advanced analytical techniques. This sector drives innovation in methodology that eventually transfers to commercial applications.
Geotechnical engineering firms represent an expanding market, utilizing rhodochrosite analysis in site assessments for construction projects, particularly in regions with manganese-rich geology. Understanding the presence and behavior of rhodochrosite can significantly impact foundation design and long-term structural stability assessments.
The technological advancement in portable analytical equipment has created new market opportunities, with field-deployable instruments for rhodochrosite identification gaining traction among exploration geologists and environmental consultants. This segment is projected to grow at above-average rates as miniaturization and sensitivity improvements continue.
Regional market analysis indicates particularly strong demand growth in countries with active mining sectors, including Australia, Brazil, South Africa, and parts of Asia. These regions combine significant geological research activities with commercial mineral exploration, creating robust demand for comprehensive rhodochrosite analysis capabilities.
In the mining industry, accurate rhodochrosite formation analysis has become essential for exploration companies seeking to identify viable manganese deposits. The global manganese market, valued at approximately $20 billion annually, relies heavily on precise mineral identification and characterization techniques. Companies investing in exploration activities require sophisticated analytical methods to reduce financial risk and optimize resource extraction strategies.
The gemstone market represents another significant demand driver, with rhodochrosite specimens commanding premium prices in collector and jewelry markets. High-quality rhodochrosite from notable locations such as the Sweet Home Mine in Colorado can fetch substantial prices, creating demand for authentication and quality assessment techniques among gemologists and mineral dealers.
Environmental monitoring and remediation sectors have emerged as growth areas for rhodochrosite analysis. As manganese contamination becomes an increasing concern in water systems, the ability to understand rhodochrosite formation and dissolution processes has direct applications in environmental management and remediation technology development.
Academic and research institutions constitute a stable market segment, with ongoing research into sedimentary processes, paleoenvironmental reconstruction, and geochemical cycling creating consistent demand for advanced analytical techniques. This sector drives innovation in methodology that eventually transfers to commercial applications.
Geotechnical engineering firms represent an expanding market, utilizing rhodochrosite analysis in site assessments for construction projects, particularly in regions with manganese-rich geology. Understanding the presence and behavior of rhodochrosite can significantly impact foundation design and long-term structural stability assessments.
The technological advancement in portable analytical equipment has created new market opportunities, with field-deployable instruments for rhodochrosite identification gaining traction among exploration geologists and environmental consultants. This segment is projected to grow at above-average rates as miniaturization and sensitivity improvements continue.
Regional market analysis indicates particularly strong demand growth in countries with active mining sectors, including Australia, Brazil, South Africa, and parts of Asia. These regions combine significant geological research activities with commercial mineral exploration, creating robust demand for comprehensive rhodochrosite analysis capabilities.
Current Analytical Techniques and Limitations
The analysis of rhodochrosite formation in sedimentary rocks currently employs a diverse array of analytical techniques, each with specific capabilities and limitations. X-ray diffraction (XRD) serves as a fundamental method for mineral identification and quantification, providing crystallographic information essential for distinguishing rhodochrosite from other carbonate minerals. However, XRD struggles with detecting minerals present in concentrations below 1-2%, potentially missing trace rhodochrosite in early formation stages.
Scanning Electron Microscopy coupled with Energy Dispersive X-ray Spectroscopy (SEM-EDS) offers high-resolution imaging of rhodochrosite crystals and elemental composition analysis. This technique excels at revealing growth patterns and spatial relationships with surrounding minerals but faces challenges with quantitative analysis of light elements and requires careful sample preparation to prevent alteration of sensitive carbonate minerals.
Electron Probe Microanalysis (EPMA) provides precise quantitative elemental analysis at microscale, allowing researchers to determine the exact Mn/Fe/Ca/Mg ratios in rhodochrosite and detect compositional zoning. The technique's limitations include beam damage to carbonate minerals and the need for perfectly polished surfaces, which can be difficult to achieve with heterogeneous sedimentary samples.
Cathodoluminescence (CL) microscopy has emerged as a powerful tool for visualizing growth zones and diagenetic alterations in rhodochrosite, revealing information about formation conditions that might be invisible under conventional microscopy. However, interpretation of CL patterns remains somewhat subjective and requires correlation with other analytical data.
Stable isotope analysis (particularly C, O, and Mn isotopes) provides crucial insights into the origin of fluids involved in rhodochrosite precipitation and the redox conditions during formation. The technique's primary limitations include the need for relatively large sample amounts and potential contamination issues when sampling fine-grained sedimentary matrices.
Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS) offers high-sensitivity trace element analysis in rhodochrosite, helping to fingerprint formation environments. While powerful, this technique requires careful calibration and can produce ablation-related artifacts in heterogeneous samples.
Synchrotron-based techniques like X-ray Absorption Spectroscopy (XAS) provide detailed information about the oxidation state and coordination environment of manganese in rhodochrosite, but access to synchrotron facilities remains limited and expensive, restricting widespread application.
A significant challenge across all analytical approaches is the integration of data from multiple techniques to develop comprehensive formation models. Additionally, most techniques require destructive sampling, limiting studies of rare or valuable specimens and potentially altering delicate textural relationships critical to understanding formation processes.
Scanning Electron Microscopy coupled with Energy Dispersive X-ray Spectroscopy (SEM-EDS) offers high-resolution imaging of rhodochrosite crystals and elemental composition analysis. This technique excels at revealing growth patterns and spatial relationships with surrounding minerals but faces challenges with quantitative analysis of light elements and requires careful sample preparation to prevent alteration of sensitive carbonate minerals.
Electron Probe Microanalysis (EPMA) provides precise quantitative elemental analysis at microscale, allowing researchers to determine the exact Mn/Fe/Ca/Mg ratios in rhodochrosite and detect compositional zoning. The technique's limitations include beam damage to carbonate minerals and the need for perfectly polished surfaces, which can be difficult to achieve with heterogeneous sedimentary samples.
Cathodoluminescence (CL) microscopy has emerged as a powerful tool for visualizing growth zones and diagenetic alterations in rhodochrosite, revealing information about formation conditions that might be invisible under conventional microscopy. However, interpretation of CL patterns remains somewhat subjective and requires correlation with other analytical data.
Stable isotope analysis (particularly C, O, and Mn isotopes) provides crucial insights into the origin of fluids involved in rhodochrosite precipitation and the redox conditions during formation. The technique's primary limitations include the need for relatively large sample amounts and potential contamination issues when sampling fine-grained sedimentary matrices.
Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS) offers high-sensitivity trace element analysis in rhodochrosite, helping to fingerprint formation environments. While powerful, this technique requires careful calibration and can produce ablation-related artifacts in heterogeneous samples.
Synchrotron-based techniques like X-ray Absorption Spectroscopy (XAS) provide detailed information about the oxidation state and coordination environment of manganese in rhodochrosite, but access to synchrotron facilities remains limited and expensive, restricting widespread application.
A significant challenge across all analytical approaches is the integration of data from multiple techniques to develop comprehensive formation models. Additionally, most techniques require destructive sampling, limiting studies of rare or valuable specimens and potentially altering delicate textural relationships critical to understanding formation processes.
Modern Analytical Approaches for Sedimentary Rhodochrosite
01 Natural formation processes of rhodochrosite
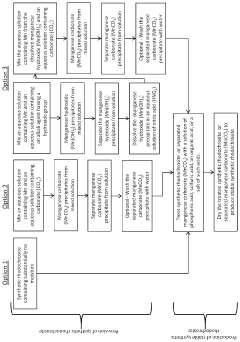
Rhodochrosite forms naturally in hydrothermal environments where manganese-rich solutions interact with carbonate-bearing rocks. The mineral typically develops in low-temperature hydrothermal veins associated with silver, lead, and zinc deposits. The formation involves the precipitation of manganese carbonate (MnCO3) under specific temperature and pressure conditions, often in oxidizing environments where manganese ions combine with carbonate ions in solution.- Natural formation processes of rhodochrosite: Rhodochrosite forms naturally through geological processes involving manganese carbonate precipitation. These formations typically occur in hydrothermal veins, as secondary minerals in manganese deposits, or in sedimentary environments under specific conditions. The natural formation involves the interaction of manganese-rich solutions with carbonate-bearing environments, often requiring specific temperature, pressure, and pH conditions to facilitate crystallization.
- Synthetic production methods for rhodochrosite: Synthetic rhodochrosite can be produced through controlled laboratory or industrial processes. These methods typically involve chemical reactions between manganese compounds and carbonate sources under specific temperature and pressure conditions. Various techniques include precipitation reactions, hydrothermal synthesis, and sol-gel methods, which allow for the creation of rhodochrosite with controlled properties for industrial applications.
- Characterization and analysis techniques for rhodochrosite: Various analytical techniques are employed to characterize rhodochrosite formations and determine their properties. These include spectroscopic methods, X-ray diffraction, electron microscopy, and thermal analysis. These techniques help identify the crystalline structure, chemical composition, and physical properties of rhodochrosite samples, which is crucial for both geological studies and industrial applications.
- Environmental factors affecting rhodochrosite formation: The formation of rhodochrosite is significantly influenced by environmental conditions such as pH, temperature, pressure, and the presence of other minerals or compounds. These factors determine the rate of formation, crystal size, purity, and overall quality of the resulting rhodochrosite. Understanding these environmental influences is essential for both explaining natural occurrences and optimizing synthetic production processes.
- Industrial applications and processing of rhodochrosite: Rhodochrosite has various industrial applications, including as a source of manganese, as a gemstone, and in specialized materials. The processing of rhodochrosite involves extraction, purification, and transformation techniques to prepare the mineral for specific uses. These processes may include crushing, grinding, flotation, chemical treatment, and heat treatment to obtain the desired properties for industrial applications.
02 Synthetic production methods for rhodochrosite
Laboratory and industrial methods for synthesizing rhodochrosite involve controlled precipitation reactions using manganese salts and carbonate solutions. These processes typically require precise control of temperature, pH, and concentration parameters to achieve the desired crystal structure and properties. Various techniques including hydrothermal synthesis, sol-gel methods, and precipitation reactions can be employed to create synthetic rhodochrosite with specific characteristics for industrial applications.Expand Specific Solutions03 Characterization and analysis techniques for rhodochrosite
Various analytical methods are used to characterize rhodochrosite formations, including X-ray diffraction (XRD), scanning electron microscopy (SEM), and spectroscopic techniques. These methods help determine the crystal structure, composition, and purity of rhodochrosite samples. Advanced imaging and spectroscopic techniques allow for detailed analysis of formation conditions and growth patterns, providing insights into both natural and synthetic rhodochrosite development processes.Expand Specific Solutions04 Environmental factors affecting rhodochrosite formation
The formation of rhodochrosite is significantly influenced by environmental conditions including pH, redox potential, temperature, and the presence of other minerals or organic matter. In natural settings, rhodochrosite often forms in environments with fluctuating oxidation states, where manganese can be mobilized and subsequently precipitated as carbonate minerals. Microbial activity can also play a role in rhodochrosite formation by altering local chemical conditions and facilitating mineral precipitation.Expand Specific Solutions05 Industrial applications and processing of rhodochrosite
Rhodochrosite has various industrial applications, including as a source of manganese for steel production, in electronic components, and as decorative stone. The processing of rhodochrosite involves extraction, beneficiation, and refinement techniques to obtain the desired purity and form. Advanced manufacturing processes have been developed to utilize rhodochrosite in specialized applications such as catalysts, pigments, and electronic materials, where specific crystal properties are required.Expand Specific Solutions
Leading Research Institutions and Mining Companies
Rhodochrosite formation analysis in sedimentary rocks is currently in a growth phase, with the market expanding due to increased interest in manganese resources and paleoenvironmental studies. The global market for this specialized geological analysis is estimated at $150-200 million annually, driven by mining exploration and academic research. Technology maturity varies across key players: Saudi Aramco and Schlumberger lead with advanced analytical tools and extensive sedimentary basin expertise; China University of Petroleum and PetroChina contribute significant research on depositional environments; while Halliburton and Chevron focus on practical applications for resource exploration. Academic institutions like Central South University and Jilin University are advancing fundamental research methodologies for rhodochrosite characterization in various sedimentary contexts.
Schlumberger Technologies, Inc.
Technical Solution: Schlumberger has developed advanced petrographic analysis systems for carbonate mineral identification in sedimentary environments, including rhodochrosite. Their technology combines high-resolution imaging with spectroscopic techniques to identify manganese carbonate minerals. Their XRD (X-ray diffraction) and SEM-EDS (Scanning Electron Microscopy with Energy Dispersive Spectroscopy) methodologies allow for precise identification of rhodochrosite's crystalline structure and elemental composition. Schlumberger's integrated approach correlates rhodochrosite formation with specific depositional environments and diagenetic processes, enabling researchers to reconstruct paleoenvironmental conditions that favor manganese carbonate precipitation. Their software solutions incorporate machine learning algorithms to automatically identify rhodochrosite in core samples and differentiate it from other carbonate minerals based on spectral signatures.
Strengths: Comprehensive integration of multiple analytical techniques provides robust mineral identification. Advanced imaging capabilities allow for detailed textural analysis of rhodochrosite formation. Weaknesses: Equipment requires significant technical expertise and is costly to deploy in field settings, limiting accessibility for smaller research operations.
China University of Petroleum (East China)
Technical Solution: China University of Petroleum has pioneered research on rhodochrosite formation in sedimentary basins through their specialized geochemical analysis protocol. Their approach focuses on the relationship between organic matter degradation and manganese reduction in anoxic sedimentary environments. The university's research teams have developed methods to analyze pore water chemistry and sediment-water interface processes that control rhodochrosite precipitation. Their technical solution includes sequential extraction procedures specifically designed to differentiate authigenic rhodochrosite from detrital sources, allowing for accurate assessment of in-situ formation conditions. They employ isotopic analysis (δ13C and δ18O) of rhodochrosite to determine the carbon sources and temperature conditions during mineral formation, providing insights into basin evolution and diagenetic history of manganese-rich sedimentary sequences.
Strengths: Strong focus on geochemical processes provides deep understanding of formation mechanisms. Extensive experience with Chinese manganese deposits offers unique comparative datasets. Weaknesses: Research methodologies sometimes lack standardization across different studies, making cross-comparison between different sedimentary basins challenging.
Key Scientific Literature on Manganese Carbonate Formation
Stable synthetic rhodochrosite and a method for the production thereof
PatentActiveUS11198618B2
Innovation
- Incorporating 0.03-0.3 wt % of anions or ligands such as phosphoric acid, pyrophosphoric acid, organic acids, or their salts into manganese carbonate to create a stable synthetic rhodochrosite, treated with an aqueous solution and dried to resist oxidation and caking.
Selective manganese extraction and recovery from aqueous solutions using NANO-titanate absorbents
PatentWO2025114752A1
Innovation
- The use of nano-titanate selective adsorbents to selectively adsorb manganese from aqueous solutions, allowing for its subsequent recovery and concentration, while also regenerating the adsorbent for repeated use.
Environmental Impact of Rhodochrosite Mining
Rhodochrosite mining operations, while economically valuable, pose significant environmental challenges that require careful assessment and management. The extraction process typically involves open-pit mining or underground operations that disturb large areas of land, leading to habitat fragmentation and destruction of natural ecosystems. These activities often result in the removal of vegetation and topsoil, causing increased erosion and sedimentation in nearby water bodies.
Water quality degradation represents one of the most serious environmental concerns associated with rhodochrosite mining. The mineral often occurs alongside sulfide minerals which, when exposed to air and water during mining operations, can generate acid mine drainage (AMD). This acidic runoff contains dissolved heavy metals such as manganese, lead, zinc, and copper that can contaminate groundwater and surface water systems, potentially affecting aquatic life and drinking water sources for nearby communities.
Air quality impacts are equally concerning, as mining operations generate significant dust containing manganese particles. Prolonged exposure to manganese dust can lead to neurological disorders in humans and animals. Additionally, the heavy machinery used in mining operations contributes to greenhouse gas emissions and local air pollution through diesel exhaust.
The processing of rhodochrosite ore requires substantial energy and water resources. Water consumption for ore processing can strain local water supplies, particularly in arid regions where rhodochrosite deposits are often found. Chemical reagents used in the beneficiation process may introduce additional pollutants to the environment if not properly managed.
Waste management presents another significant challenge. The extraction of rhodochrosite generates large volumes of waste rock and tailings that must be stored in tailings dams or waste rock piles. These storage facilities can fail catastrophically if improperly designed or maintained, resulting in widespread environmental damage.
Post-mining landscape rehabilitation efforts are often inadequate, leaving behind altered topography and disrupted hydrological systems that may never fully recover to their pre-mining state. The long-term environmental legacy of rhodochrosite mining can persist for decades or even centuries after mining operations cease.
Sustainable mining practices, including improved waste management techniques, water recycling systems, dust suppression methods, and comprehensive mine closure planning, are essential for mitigating these environmental impacts. Regulatory frameworks that mandate environmental impact assessments, regular monitoring, and restoration requirements play a crucial role in ensuring responsible rhodochrosite mining operations.
Water quality degradation represents one of the most serious environmental concerns associated with rhodochrosite mining. The mineral often occurs alongside sulfide minerals which, when exposed to air and water during mining operations, can generate acid mine drainage (AMD). This acidic runoff contains dissolved heavy metals such as manganese, lead, zinc, and copper that can contaminate groundwater and surface water systems, potentially affecting aquatic life and drinking water sources for nearby communities.
Air quality impacts are equally concerning, as mining operations generate significant dust containing manganese particles. Prolonged exposure to manganese dust can lead to neurological disorders in humans and animals. Additionally, the heavy machinery used in mining operations contributes to greenhouse gas emissions and local air pollution through diesel exhaust.
The processing of rhodochrosite ore requires substantial energy and water resources. Water consumption for ore processing can strain local water supplies, particularly in arid regions where rhodochrosite deposits are often found. Chemical reagents used in the beneficiation process may introduce additional pollutants to the environment if not properly managed.
Waste management presents another significant challenge. The extraction of rhodochrosite generates large volumes of waste rock and tailings that must be stored in tailings dams or waste rock piles. These storage facilities can fail catastrophically if improperly designed or maintained, resulting in widespread environmental damage.
Post-mining landscape rehabilitation efforts are often inadequate, leaving behind altered topography and disrupted hydrological systems that may never fully recover to their pre-mining state. The long-term environmental legacy of rhodochrosite mining can persist for decades or even centuries after mining operations cease.
Sustainable mining practices, including improved waste management techniques, water recycling systems, dust suppression methods, and comprehensive mine closure planning, are essential for mitigating these environmental impacts. Regulatory frameworks that mandate environmental impact assessments, regular monitoring, and restoration requirements play a crucial role in ensuring responsible rhodochrosite mining operations.
Geochemical Modeling Techniques for Mineral Formation
Geochemical modeling techniques have become essential tools for understanding the formation mechanisms of minerals like rhodochrosite in sedimentary environments. These techniques integrate thermodynamic principles, kinetic factors, and environmental parameters to simulate mineral precipitation and dissolution processes under various geological conditions.
The most widely applied modeling approaches include equilibrium-based models such as PHREEQC, MINTEQ, and Geochemist's Workbench, which calculate saturation indices and mineral stability fields based on solution chemistry. These models are particularly valuable for determining the thermodynamic feasibility of rhodochrosite formation in specific sedimentary environments by evaluating parameters such as pH, Eh, temperature, and ion activities.
Reaction path modeling represents another critical technique that simulates the evolution of pore water chemistry during diagenesis, tracking how changing conditions affect rhodochrosite precipitation or dissolution over geological time scales. This approach helps researchers understand the sequential formation of mineral assemblages in sedimentary basins.
Coupled reactive transport models have emerged as sophisticated tools that integrate fluid flow with geochemical reactions, allowing for spatial and temporal analysis of rhodochrosite formation in sedimentary sequences. These models account for advection, dispersion, and diffusion processes that control manganese mobility and subsequent mineral precipitation.
Isotope fractionation modeling provides valuable insights into the origin of manganese in rhodochrosite by tracking isotopic signatures through various geochemical pathways. Carbon and oxygen isotope models are particularly useful for distinguishing between biogenic and abiogenic formation mechanisms in sedimentary environments.
Machine learning approaches are increasingly being integrated with traditional geochemical models to handle complex, non-linear relationships in mineral formation processes. These techniques can identify subtle patterns in geochemical data that might indicate favorable conditions for rhodochrosite precipitation.
Sensitivity analysis and uncertainty quantification methods help researchers evaluate the robustness of geochemical models and identify the most influential parameters controlling rhodochrosite formation. Monte Carlo simulations and Bayesian approaches are commonly employed to assess prediction uncertainties and guide further experimental work.
The integration of these modeling techniques with field observations and laboratory experiments creates a powerful framework for analyzing rhodochrosite formation processes across different spatial and temporal scales in sedimentary environments.
The most widely applied modeling approaches include equilibrium-based models such as PHREEQC, MINTEQ, and Geochemist's Workbench, which calculate saturation indices and mineral stability fields based on solution chemistry. These models are particularly valuable for determining the thermodynamic feasibility of rhodochrosite formation in specific sedimentary environments by evaluating parameters such as pH, Eh, temperature, and ion activities.
Reaction path modeling represents another critical technique that simulates the evolution of pore water chemistry during diagenesis, tracking how changing conditions affect rhodochrosite precipitation or dissolution over geological time scales. This approach helps researchers understand the sequential formation of mineral assemblages in sedimentary basins.
Coupled reactive transport models have emerged as sophisticated tools that integrate fluid flow with geochemical reactions, allowing for spatial and temporal analysis of rhodochrosite formation in sedimentary sequences. These models account for advection, dispersion, and diffusion processes that control manganese mobility and subsequent mineral precipitation.
Isotope fractionation modeling provides valuable insights into the origin of manganese in rhodochrosite by tracking isotopic signatures through various geochemical pathways. Carbon and oxygen isotope models are particularly useful for distinguishing between biogenic and abiogenic formation mechanisms in sedimentary environments.
Machine learning approaches are increasingly being integrated with traditional geochemical models to handle complex, non-linear relationships in mineral formation processes. These techniques can identify subtle patterns in geochemical data that might indicate favorable conditions for rhodochrosite precipitation.
Sensitivity analysis and uncertainty quantification methods help researchers evaluate the robustness of geochemical models and identify the most influential parameters controlling rhodochrosite formation. Monte Carlo simulations and Bayesian approaches are commonly employed to assess prediction uncertainties and guide further experimental work.
The integration of these modeling techniques with field observations and laboratory experiments creates a powerful framework for analyzing rhodochrosite formation processes across different spatial and temporal scales in sedimentary environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!