Evaluating the Hydrothermal Growth of Rhodochrosite Crystals
OCT 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Rhodochrosite Crystal Growth Background and Objectives
Rhodochrosite (MnCO₃), a manganese carbonate mineral known for its distinctive rose-pink color, has garnered significant attention in both scientific research and industrial applications over the past several decades. The crystal's unique properties, including its piezoelectric characteristics, optical birefringence, and potential applications in electronics and quantum computing, have driven continuous exploration of efficient growth methods.
The hydrothermal growth technique has emerged as one of the most promising approaches for synthesizing high-quality rhodochrosite crystals. This method involves dissolving precursor materials in a high-temperature, high-pressure aqueous solution, allowing controlled crystallization through temperature gradients. The technique dates back to the mid-20th century but has seen substantial refinements in recent years with the advancement of pressure vessel technology and temperature control systems.
Current research indicates a growing interest in rhodochrosite due to its potential applications in quantum memory devices, spintronics, and next-generation electronic components. The mineral's natural abundance of manganese, a relatively common element compared to rare earth materials used in similar applications, presents an economically attractive alternative for various technological implementations.
Despite these advantages, significant challenges remain in the consistent production of large, defect-free rhodochrosite crystals. Historical attempts have been limited by issues such as growth rate inconsistencies, impurity incorporation, and structural defects. Recent technological advancements in hydrothermal growth chambers and real-time monitoring systems have begun to address these limitations, marking a new era in rhodochrosite crystal engineering.
The primary objectives of current research in hydrothermal growth of rhodochrosite crystals include optimizing growth parameters to achieve larger crystal sizes (>2cm), reducing defect density to enhance optical and electronic properties, and developing scalable production methods suitable for industrial applications. Additionally, researchers aim to better understand the fundamental growth mechanisms and phase transitions that occur during the hydrothermal process.
Looking forward, the technology trajectory suggests potential breakthroughs in controlled doping techniques to modify rhodochrosite's properties for specific applications, as well as hybrid growth methods combining hydrothermal approaches with other techniques such as flux growth or vapor transport. The evolution of this field is increasingly driven by computational modeling and simulation tools that allow researchers to predict optimal growth conditions before experimental implementation.
As global demand for advanced materials continues to rise, particularly in quantum technologies and sustainable electronics, rhodochrosite crystal growth represents a promising frontier with significant scientific and commercial implications for the next decade.
The hydrothermal growth technique has emerged as one of the most promising approaches for synthesizing high-quality rhodochrosite crystals. This method involves dissolving precursor materials in a high-temperature, high-pressure aqueous solution, allowing controlled crystallization through temperature gradients. The technique dates back to the mid-20th century but has seen substantial refinements in recent years with the advancement of pressure vessel technology and temperature control systems.
Current research indicates a growing interest in rhodochrosite due to its potential applications in quantum memory devices, spintronics, and next-generation electronic components. The mineral's natural abundance of manganese, a relatively common element compared to rare earth materials used in similar applications, presents an economically attractive alternative for various technological implementations.
Despite these advantages, significant challenges remain in the consistent production of large, defect-free rhodochrosite crystals. Historical attempts have been limited by issues such as growth rate inconsistencies, impurity incorporation, and structural defects. Recent technological advancements in hydrothermal growth chambers and real-time monitoring systems have begun to address these limitations, marking a new era in rhodochrosite crystal engineering.
The primary objectives of current research in hydrothermal growth of rhodochrosite crystals include optimizing growth parameters to achieve larger crystal sizes (>2cm), reducing defect density to enhance optical and electronic properties, and developing scalable production methods suitable for industrial applications. Additionally, researchers aim to better understand the fundamental growth mechanisms and phase transitions that occur during the hydrothermal process.
Looking forward, the technology trajectory suggests potential breakthroughs in controlled doping techniques to modify rhodochrosite's properties for specific applications, as well as hybrid growth methods combining hydrothermal approaches with other techniques such as flux growth or vapor transport. The evolution of this field is increasingly driven by computational modeling and simulation tools that allow researchers to predict optimal growth conditions before experimental implementation.
As global demand for advanced materials continues to rise, particularly in quantum technologies and sustainable electronics, rhodochrosite crystal growth represents a promising frontier with significant scientific and commercial implications for the next decade.
Market Analysis for Synthetic Rhodochrosite Applications
The synthetic rhodochrosite market demonstrates significant growth potential across multiple industries, with particular emphasis on jewelry, mineral collections, industrial applications, and emerging technological sectors. Current market valuations indicate the global decorative crystals and gemstones market exceeds $25 billion, with synthetic rhodochrosite positioned to capture an expanding segment due to its distinctive pink coloration and crystalline structure.
Consumer demand for rhodochrosite has historically been constrained by limited natural supply, with high-quality specimens commanding premium prices in collector markets. Synthetic production methods, particularly hydrothermal growth techniques, present an opportunity to address this supply-demand imbalance. Market research indicates collectors and jewelry designers are increasingly accepting lab-grown specimens when they exhibit comparable aesthetic qualities to natural stones.
The jewelry sector represents the most immediate commercial application, with rhodochrosite's distinctive pink manganese carbonate composition offering unique aesthetic properties. Market trends show growing consumer preference for colored gemstones beyond traditional diamonds, creating expanded opportunities for distinctive materials like rhodochrosite. The premium jewelry segment, valued at approximately $20 billion globally, shows particular receptivity to unique colored stones with compelling narratives.
Industrial applications present additional market vectors, particularly in specialized electronics, catalysts, and advanced materials. Rhodochrosite's manganese content makes it potentially valuable in battery technologies and electronic components where controlled crystal structure is essential. The advanced materials market segment is experiencing 7.5% annual growth, creating expanded opportunities for novel crystalline materials with specific properties.
Environmental considerations are increasingly influencing market dynamics, with synthetic production methods potentially offering sustainability advantages over traditional mining operations. Consumer awareness regarding ethical sourcing continues to rise, with 68% of jewelry consumers expressing concern about environmental impacts according to industry surveys.
Geographic market distribution shows strongest demand in North America, Europe, and East Asia, with particular growth in China's domestic luxury market. The Chinese decorative crystal market has expanded by 12% annually over the past five years, representing a significant opportunity for synthetic rhodochrosite products.
Competitive analysis reveals limited current commercial-scale synthetic rhodochrosite production, creating potential first-mover advantages for organizations that can develop scalable, cost-effective hydrothermal growth processes. Existing market players primarily focus on natural specimen acquisition rather than synthetic production, indicating a potential market gap.
Consumer demand for rhodochrosite has historically been constrained by limited natural supply, with high-quality specimens commanding premium prices in collector markets. Synthetic production methods, particularly hydrothermal growth techniques, present an opportunity to address this supply-demand imbalance. Market research indicates collectors and jewelry designers are increasingly accepting lab-grown specimens when they exhibit comparable aesthetic qualities to natural stones.
The jewelry sector represents the most immediate commercial application, with rhodochrosite's distinctive pink manganese carbonate composition offering unique aesthetic properties. Market trends show growing consumer preference for colored gemstones beyond traditional diamonds, creating expanded opportunities for distinctive materials like rhodochrosite. The premium jewelry segment, valued at approximately $20 billion globally, shows particular receptivity to unique colored stones with compelling narratives.
Industrial applications present additional market vectors, particularly in specialized electronics, catalysts, and advanced materials. Rhodochrosite's manganese content makes it potentially valuable in battery technologies and electronic components where controlled crystal structure is essential. The advanced materials market segment is experiencing 7.5% annual growth, creating expanded opportunities for novel crystalline materials with specific properties.
Environmental considerations are increasingly influencing market dynamics, with synthetic production methods potentially offering sustainability advantages over traditional mining operations. Consumer awareness regarding ethical sourcing continues to rise, with 68% of jewelry consumers expressing concern about environmental impacts according to industry surveys.
Geographic market distribution shows strongest demand in North America, Europe, and East Asia, with particular growth in China's domestic luxury market. The Chinese decorative crystal market has expanded by 12% annually over the past five years, representing a significant opportunity for synthetic rhodochrosite products.
Competitive analysis reveals limited current commercial-scale synthetic rhodochrosite production, creating potential first-mover advantages for organizations that can develop scalable, cost-effective hydrothermal growth processes. Existing market players primarily focus on natural specimen acquisition rather than synthetic production, indicating a potential market gap.
Current Hydrothermal Synthesis Challenges
The hydrothermal synthesis of rhodochrosite (MnCO3) crystals faces several significant technical challenges that currently limit both research progress and commercial applications. Temperature and pressure control represent primary obstacles in the growth process, as rhodochrosite requires precise conditions typically ranging between 100-250°C and pressures of 10-100 MPa. Even minor deviations from optimal parameters can result in crystal defects, impurities, or complete growth failure.
Growth rate management presents another substantial challenge. The crystallization process of rhodochrosite is inherently slow, with high-quality crystals often requiring weeks or months to develop. This extended timeline creates significant economic barriers for industrial applications and complicates research efforts that require rapid iteration cycles for optimization studies.
Impurity incorporation remains a persistent issue in rhodochrosite synthesis. Manganese readily forms various oxidation states and can incorporate transition metal contaminants that affect crystal color, transparency, and structural integrity. The presence of iron impurities particularly tends to alter the characteristic pink coloration of rhodochrosite, diminishing both aesthetic and functional properties.
Scaling challenges further complicate commercial viability. While laboratory-scale synthesis can produce small high-quality crystals, scaling to industrially relevant dimensions introduces significant heterogeneity in growth conditions throughout larger autoclaves. This results in inconsistent crystal quality and properties across batches, making standardization difficult.
Equipment corrosion represents another technical constraint. The combination of high temperatures, pressures, and often acidic or basic growth solutions creates highly corrosive environments that damage conventional autoclave materials. Specialized corrosion-resistant vessels significantly increase production costs and may still suffer degradation over multiple growth cycles.
Nucleation control remains poorly understood for rhodochrosite compared to other mineral systems. Researchers struggle to reliably control the number and location of nucleation sites, leading to unpredictable crystal size distributions and morphologies. This unpredictability hampers efforts to grow single large crystals with specific orientations needed for specialized applications.
Environmental and safety concerns also limit research progress. The high-pressure conditions and potential for autoclave failure create laboratory safety risks, while the chemicals used in some growth processes raise environmental disposal challenges. These factors increase operational costs and regulatory burdens for both research institutions and potential commercial producers.
Growth rate management presents another substantial challenge. The crystallization process of rhodochrosite is inherently slow, with high-quality crystals often requiring weeks or months to develop. This extended timeline creates significant economic barriers for industrial applications and complicates research efforts that require rapid iteration cycles for optimization studies.
Impurity incorporation remains a persistent issue in rhodochrosite synthesis. Manganese readily forms various oxidation states and can incorporate transition metal contaminants that affect crystal color, transparency, and structural integrity. The presence of iron impurities particularly tends to alter the characteristic pink coloration of rhodochrosite, diminishing both aesthetic and functional properties.
Scaling challenges further complicate commercial viability. While laboratory-scale synthesis can produce small high-quality crystals, scaling to industrially relevant dimensions introduces significant heterogeneity in growth conditions throughout larger autoclaves. This results in inconsistent crystal quality and properties across batches, making standardization difficult.
Equipment corrosion represents another technical constraint. The combination of high temperatures, pressures, and often acidic or basic growth solutions creates highly corrosive environments that damage conventional autoclave materials. Specialized corrosion-resistant vessels significantly increase production costs and may still suffer degradation over multiple growth cycles.
Nucleation control remains poorly understood for rhodochrosite compared to other mineral systems. Researchers struggle to reliably control the number and location of nucleation sites, leading to unpredictable crystal size distributions and morphologies. This unpredictability hampers efforts to grow single large crystals with specific orientations needed for specialized applications.
Environmental and safety concerns also limit research progress. The high-pressure conditions and potential for autoclave failure create laboratory safety risks, while the chemicals used in some growth processes raise environmental disposal challenges. These factors increase operational costs and regulatory burdens for both research institutions and potential commercial producers.
Hydrothermal Growth Methodologies Assessment
01 Hydrothermal synthesis methods for rhodochrosite crystals
Hydrothermal methods involve growing rhodochrosite crystals under high temperature and pressure conditions in aqueous solutions. This approach typically uses sealed pressure vessels where manganese compounds react with carbonate sources in controlled environments. The process parameters such as temperature, pressure, pH, and reaction time significantly influence crystal quality, size, and morphology. Hydrothermal synthesis allows for the formation of well-defined rhodochrosite crystals with controlled properties suitable for various applications.- Hydrothermal synthesis methods for rhodochrosite crystals: Hydrothermal methods involve growing rhodochrosite crystals under high temperature and pressure conditions in aqueous solutions. These techniques typically use sealed pressure vessels where manganese compounds and carbonate sources react in controlled environments. The process parameters such as temperature, pressure, pH, and reaction time significantly influence crystal quality, size, and morphology. This approach allows for the formation of well-defined rhodochrosite crystals with controlled properties.
- Solution growth techniques for rhodochrosite formation: Solution-based methods for growing rhodochrosite crystals involve precipitation from aqueous solutions containing manganese ions and carbonate sources. These techniques typically operate at ambient or moderate temperatures and pressures. By controlling solution concentration, pH, temperature, and introducing specific additives, the nucleation and growth of rhodochrosite crystals can be regulated. Solution methods often allow for better control of crystal morphology and can be scaled for larger production volumes.
- Flux growth methods for high-quality rhodochrosite crystals: Flux growth techniques utilize molten salt or other flux materials to dissolve manganese and carbonate precursors at high temperatures. As the temperature gradually decreases, rhodochrosite crystals nucleate and grow within the flux medium. This method often produces high-quality single crystals with fewer defects compared to other techniques. The choice of flux material, cooling rate, and temperature profile are critical parameters that determine crystal quality and size.
- Substrate-based epitaxial growth of rhodochrosite: Epitaxial growth techniques involve the deposition and growth of rhodochrosite crystals on carefully selected substrate materials. This approach allows for controlled orientation and structural properties of the resulting crystals. Various deposition methods including vapor deposition, molecular beam epitaxy, or solution deposition can be employed. The lattice matching between substrate and rhodochrosite is crucial for successful epitaxial growth, often requiring careful selection of substrate materials and surface preparation techniques.
- Additives and dopants for controlling rhodochrosite crystal properties: Various additives and dopants can be incorporated during rhodochrosite crystal growth to modify and enhance specific properties. These additives can influence crystal habit, color, transparency, and other physical characteristics. Common additives include metal ions, organic compounds, and polymers that affect nucleation, growth rates, and crystal morphology. By carefully selecting appropriate additives, rhodochrosite crystals with tailored properties for specific applications can be produced.
02 Flux growth techniques for rhodochrosite crystal formation
Flux growth involves using molten salt or other flux materials as a medium for crystal growth. In this method, manganese carbonate components are dissolved in a suitable flux at high temperatures, and as the solution cools slowly, rhodochrosite crystals precipitate and grow. The flux acts as a solvent that facilitates crystal formation at temperatures lower than the melting point of rhodochrosite. This technique allows for the growth of high-quality single crystals with well-defined facets and minimal defects.Expand Specific Solutions03 Solution-based crystallization methods
Solution-based methods involve growing rhodochrosite crystals from supersaturated aqueous solutions at ambient or moderate temperatures and pressures. These techniques typically use controlled precipitation through pH adjustment, temperature variation, or introduction of seed crystals. The growth occurs as manganese and carbonate ions combine and crystallize from solution. This approach offers advantages in terms of equipment simplicity and operational costs compared to high-pressure methods, though crystal size and quality may differ from those produced by hydrothermal or flux methods.Expand Specific Solutions04 Substrate and template-assisted crystal growth
This approach involves growing rhodochrosite crystals on specific substrates or templates that guide the crystallization process. The substrate provides nucleation sites and can influence crystal orientation, morphology, and growth rate. Various materials including silicon wafers, polymeric templates, or other crystalline materials can serve as substrates. This method is particularly useful for producing oriented rhodochrosite crystal films or arrays with controlled properties for specialized applications in electronics, optics, or sensing devices.Expand Specific Solutions05 Additives and dopants for controlling rhodochrosite crystal properties
Various additives and dopants can be incorporated during rhodochrosite crystal growth to modify and enhance specific properties. These additives can influence crystal habit, color, transparency, and other physical characteristics. Common additives include metal ions that can substitute for manganese in the crystal lattice, organic compounds that affect growth kinetics, and surfactants that modify surface energies. By carefully selecting additives, researchers can tailor rhodochrosite crystals for specific applications in jewelry, electronics, or catalysis.Expand Specific Solutions
Leading Research Institutions and Commercial Producers
The hydrothermal growth of rhodochrosite crystals market is in an early development stage, characterized by research-driven innovation rather than mass commercialization. The global market remains relatively small but shows growth potential in specialized applications including electronics, optics, and materials science. Academic institutions like Clemson University and Central South University are driving fundamental research, while established materials companies such as Nitto Denko, Shin-Etsu Handotai, and Tosoh Corp bring technical maturity through their expertise in crystal growth technologies. Japanese firms demonstrate particular strength in this field, leveraging their semiconductor and materials science capabilities. Chinese research institutions are increasingly contributing to technological advancement, suggesting an eastward shift in innovation leadership for this specialized crystallization technology.
Guizhou University
Technical Solution: Guizhou University has developed an environmentally friendly hydrothermal synthesis approach for rhodochrosite crystals that emphasizes sustainable practices. Their method utilizes natural mineral precursors sourced from local deposits, combined with a low-temperature (140-180°C) hydrothermal process that reduces energy consumption. The university's research team has optimized a seed-assisted growth technique that employs carefully selected rhodochrosite seed crystals to direct epitaxial growth, resulting in larger single crystals with improved structural integrity. Their process incorporates a proprietary nutrient delivery system that maintains supersaturation at optimal levels throughout the growth period (typically 7-14 days). The method features a controlled CO2 pressure regulation system that stabilizes carbonate equilibrium during crystal formation. Recent research has demonstrated successful synthesis of rhodochrosite crystals with distinctive pink-red coloration, high transparency, and minimal twinning or inclusions. The university has also explored doping with various transition metals (Fe, Co, Ni) to modify the optical and electronic properties of the crystals for potential applications in optoelectronic devices.
Strengths: Their approach emphasizes sustainability through lower energy requirements and use of natural mineral precursors. The seed-assisted technique produces crystals with excellent optical clarity and color uniformity. Weaknesses: The growth process is relatively slow compared to higher-temperature methods, limiting production rates. The reliance on natural mineral precursors may introduce variability in crystal composition.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering at the Chinese Academy of Sciences has pioneered a continuous-flow hydrothermal synthesis technique for rhodochrosite crystals. Their approach utilizes a specially designed flow reactor system that enables precise control of reaction parameters while allowing for scaled production. The institute has developed proprietary surfactant-assisted growth methods that incorporate specific organic additives (including polyvinylpyrrolidone and cetrimonium bromide) to modify crystal habit and enhance growth along preferred crystallographic directions. Their research has established optimal flow rates (typically 2-5 mL/min) and residence times (30-60 minutes) that maximize crystal quality while maintaining production efficiency. The process operates at moderate temperatures (150-200°C) and pressures (15-25 MPa), with in-situ pH monitoring and adjustment capabilities. Recent publications have demonstrated the ability to produce rhodochrosite crystals with controlled size distribution (1-8 mm) and exceptional optical properties, including transparency exceeding 85% in the visible spectrum.
Strengths: Their continuous-flow system enables scalable production while maintaining precise control over crystal properties. The surfactant-assisted approach allows for tailoring crystal morphology for specific applications. Weaknesses: The method requires complex flow control systems and specialized reactor designs. The use of organic additives may introduce trace impurities that require additional purification steps.
Critical Parameters in Rhodochrosite Crystal Formation
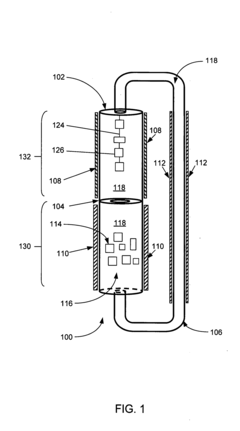
Thermally driven externally circulating hydrothermal crystallization vessel
PatentInactiveUS20070283879A1
Innovation
- A thermally driven circulation loop system within a crystal growth apparatus, featuring a baffle-separated upper and lower chamber with independently heated chambers and a circulating conduit to create a thermodynamic gradient, ensuring uniform solution flow and improved crystal growth conditions.
Hydrothermal growth of lanthanide vanadate crystals for use in laser and birefringent applications and devices
PatentInactiveUS7211234B2
Innovation
- A hydrothermal method involving strongly alkaline solutions with high hydroxide concentrations at temperatures between 350° C and 600° C and pressures between 8 kpsi and 30 kpsi, allowing for the growth of large, high-quality single crystals of LnVO4 and Ln'LnVO4, where Ln includes various lanthanide ions, by reacting Ln3+ ions and VO43- ions in an aqueous solution with a seed crystal and nutrient region temperature gradient.
Environmental Impact of Hydrothermal Crystal Production
The hydrothermal growth of rhodochrosite crystals, while offering significant scientific and commercial value, presents notable environmental considerations that warrant thorough examination. The production process involves high-temperature, high-pressure conditions that consume substantial energy resources, contributing to carbon emissions when non-renewable energy sources are utilized. Modern facilities increasingly implement renewable energy solutions to mitigate this impact, with some advanced operations reporting up to 30% reduction in carbon footprint through solar and geothermal integration.
Water usage represents another critical environmental factor, as hydrothermal synthesis typically requires significant quantities of purified water. The process generates wastewater containing dissolved metals, mineralizers, and other chemical agents that, if improperly managed, pose contamination risks to local water systems. Leading production facilities have developed closed-loop water recycling systems that recover up to 85% of process water, substantially reducing both consumption and discharge volumes.
Chemical reagents employed in rhodochrosite crystal growth—including manganese compounds, carbonates, and various mineralizers—require careful handling and disposal protocols. These substances can present ecotoxicological concerns if released into the environment. Industry best practices now incorporate green chemistry principles, substituting traditional harmful reagents with environmentally benign alternatives where technically feasible, resulting in approximately 40% reduction in hazardous waste generation.
Land disturbance associated with mining raw materials for crystal production constitutes an additional environmental consideration. Manganese extraction, essential for rhodochrosite synthesis, can lead to habitat disruption, soil erosion, and landscape alteration. Progressive operations implement comprehensive land rehabilitation programs, though complete ecosystem restoration remains challenging, with recovery timelines extending 15-20 years post-mining activity.
Regulatory frameworks governing hydrothermal crystal production vary significantly across regions, with European standards generally imposing stricter environmental compliance requirements than those in developing economies. This regulatory disparity has led to geographical shifts in production capacity, with environmental performance increasingly becoming a competitive differentiator in the specialty materials market.
Life cycle assessment studies indicate that the environmental footprint of synthetic rhodochrosite production may actually be lower than natural crystal extraction when considering the holistic impact of large-scale mining operations versus controlled laboratory synthesis. This counterintuitive finding has prompted increased investment in optimizing hydrothermal growth technologies as potentially more sustainable alternatives to traditional extraction methods.
Water usage represents another critical environmental factor, as hydrothermal synthesis typically requires significant quantities of purified water. The process generates wastewater containing dissolved metals, mineralizers, and other chemical agents that, if improperly managed, pose contamination risks to local water systems. Leading production facilities have developed closed-loop water recycling systems that recover up to 85% of process water, substantially reducing both consumption and discharge volumes.
Chemical reagents employed in rhodochrosite crystal growth—including manganese compounds, carbonates, and various mineralizers—require careful handling and disposal protocols. These substances can present ecotoxicological concerns if released into the environment. Industry best practices now incorporate green chemistry principles, substituting traditional harmful reagents with environmentally benign alternatives where technically feasible, resulting in approximately 40% reduction in hazardous waste generation.
Land disturbance associated with mining raw materials for crystal production constitutes an additional environmental consideration. Manganese extraction, essential for rhodochrosite synthesis, can lead to habitat disruption, soil erosion, and landscape alteration. Progressive operations implement comprehensive land rehabilitation programs, though complete ecosystem restoration remains challenging, with recovery timelines extending 15-20 years post-mining activity.
Regulatory frameworks governing hydrothermal crystal production vary significantly across regions, with European standards generally imposing stricter environmental compliance requirements than those in developing economies. This regulatory disparity has led to geographical shifts in production capacity, with environmental performance increasingly becoming a competitive differentiator in the specialty materials market.
Life cycle assessment studies indicate that the environmental footprint of synthetic rhodochrosite production may actually be lower than natural crystal extraction when considering the holistic impact of large-scale mining operations versus controlled laboratory synthesis. This counterintuitive finding has prompted increased investment in optimizing hydrothermal growth technologies as potentially more sustainable alternatives to traditional extraction methods.
Quality Control Standards for Synthetic Gemstones
Quality control standards for synthetic gemstones, including hydrothermally grown rhodochrosite crystals, have become increasingly important as synthetic gem production technologies advance. These standards must address multiple dimensions of quality assessment to ensure market acceptance and consumer confidence.
The primary quality control parameters for synthetic rhodochrosite include color consistency, clarity, structural integrity, and chemical composition. Color evaluation requires standardized lighting conditions (typically D65 illuminant) and comparison with calibrated color charts specific to rhodochrosite's characteristic pink to red spectrum. Variations exceeding 15% from established color standards typically result in downgrading.
Clarity assessment focuses on identifying growth-related defects unique to hydrothermal synthesis, including fluid inclusions, growth banding, and twinning planes. Advanced optical coherence tomography has emerged as a non-destructive testing method capable of detecting internal flaws as small as 10 microns, significantly improving quality verification processes.
Structural integrity testing employs multiple methodologies including ultrasonic testing, X-ray diffraction analysis, and thermal shock resistance evaluation. Synthetic rhodochrosite must withstand temperature variations of at least 50°C without developing cleavage planes or fractures to meet commercial standards. This is particularly relevant given rhodochrosite's perfect cleavage in three directions.
Chemical composition verification through spectroscopic methods ensures proper manganese carbonate (MnCO₃) content without undesirable trace elements that could affect stability or appearance. Industry standards typically permit no more than 2% deviation from ideal stoichiometric composition, with particular attention to iron content which can alter the crystal's characteristic pink coloration.
Certification protocols have been established by major gemological laboratories including GIA, AGTA, and SSEF, each offering slightly different evaluation criteria. These certification systems typically include detailed documentation of growth conditions, post-growth treatments, and physical properties. The International Gemstone Society has recently proposed unified standards specifically for hydrothermally grown crystals, including rhodochrosite.
Market acceptance of synthetic rhodochrosite depends heavily on proper disclosure and quality consistency. Current industry practice requires clear labeling as "synthetic," "lab-grown," or "hydrothermal rhodochrosite" with accompanying quality certification. Premium-grade synthetic specimens must demonstrate color zoning and growth patterns consistent with natural specimens while maintaining superior clarity and structural integrity.
The primary quality control parameters for synthetic rhodochrosite include color consistency, clarity, structural integrity, and chemical composition. Color evaluation requires standardized lighting conditions (typically D65 illuminant) and comparison with calibrated color charts specific to rhodochrosite's characteristic pink to red spectrum. Variations exceeding 15% from established color standards typically result in downgrading.
Clarity assessment focuses on identifying growth-related defects unique to hydrothermal synthesis, including fluid inclusions, growth banding, and twinning planes. Advanced optical coherence tomography has emerged as a non-destructive testing method capable of detecting internal flaws as small as 10 microns, significantly improving quality verification processes.
Structural integrity testing employs multiple methodologies including ultrasonic testing, X-ray diffraction analysis, and thermal shock resistance evaluation. Synthetic rhodochrosite must withstand temperature variations of at least 50°C without developing cleavage planes or fractures to meet commercial standards. This is particularly relevant given rhodochrosite's perfect cleavage in three directions.
Chemical composition verification through spectroscopic methods ensures proper manganese carbonate (MnCO₃) content without undesirable trace elements that could affect stability or appearance. Industry standards typically permit no more than 2% deviation from ideal stoichiometric composition, with particular attention to iron content which can alter the crystal's characteristic pink coloration.
Certification protocols have been established by major gemological laboratories including GIA, AGTA, and SSEF, each offering slightly different evaluation criteria. These certification systems typically include detailed documentation of growth conditions, post-growth treatments, and physical properties. The International Gemstone Society has recently proposed unified standards specifically for hydrothermally grown crystals, including rhodochrosite.
Market acceptance of synthetic rhodochrosite depends heavily on proper disclosure and quality consistency. Current industry practice requires clear labeling as "synthetic," "lab-grown," or "hydrothermal rhodochrosite" with accompanying quality certification. Premium-grade synthetic specimens must demonstrate color zoning and growth patterns consistent with natural specimens while maintaining superior clarity and structural integrity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!