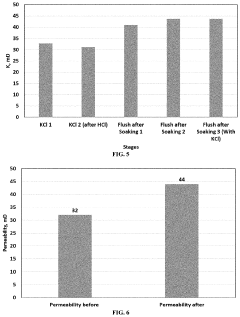
Comparing Rhodochrosite and Fluorite in Acid Reactions
OCT 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Rhodochrosite and Fluorite Acid Reaction Background
The study of mineral reactions with acids represents a significant area of research in geochemistry and materials science. Rhodochrosite (MnCO₃) and fluorite (CaF₂) are two minerals with distinct chemical compositions and crystalline structures that exhibit markedly different behaviors when exposed to acidic environments. These differences have profound implications for various industrial applications, environmental processes, and geological studies.
Rhodochrosite, a manganese carbonate mineral with its characteristic pink to red coloration, has been known since ancient times and was first scientifically described in the 18th century. Its reactivity with acids stems from its carbonate composition, making it particularly susceptible to acid dissolution through the release of carbon dioxide. This property has been extensively documented since the early development of chemical mineralogy.
Fluorite, conversely, represents a calcium fluoride mineral that has been utilized since antiquity for ornamental purposes and later for industrial applications. Its interaction with acids follows a different chemical pathway, primarily involving the formation of hydrofluoric acid when reacted with strong acids, a property recognized in the early 19th century that revolutionized glass etching techniques.
The scientific understanding of these acid-mineral interactions has evolved significantly over the past century. Early investigations focused primarily on qualitative observations, while modern research employs sophisticated analytical techniques including X-ray diffraction, scanning electron microscopy, and spectroscopic methods to characterize reaction mechanisms at the molecular level.
The technological significance of these reactions has grown exponentially with industrial development. Rhodochrosite's acid dissolution properties have become crucial in hydrometallurgical processes for manganese extraction, while fluorite's acid reactions are fundamental to the production of hydrofluoric acid, a critical component in numerous industrial applications including semiconductor manufacturing and uranium processing.
Environmental considerations have added another dimension to this field of study. The acid dissolution of rhodochrosite in mine drainage can release manganese into water systems, while fluorite reactions with acidic groundwater may contribute to fluoride contamination issues. These environmental implications have driven research toward understanding reaction kinetics under various pH conditions and developing remediation strategies.
Recent technological advances have enabled more precise control and utilization of these acid-mineral reactions. Innovations in reaction engineering have optimized manganese extraction from rhodochrosite while minimizing environmental impact, and new methodologies for fluorite processing have enhanced efficiency in hydrofluoric acid production while addressing safety concerns associated with this hazardous substance.
Rhodochrosite, a manganese carbonate mineral with its characteristic pink to red coloration, has been known since ancient times and was first scientifically described in the 18th century. Its reactivity with acids stems from its carbonate composition, making it particularly susceptible to acid dissolution through the release of carbon dioxide. This property has been extensively documented since the early development of chemical mineralogy.
Fluorite, conversely, represents a calcium fluoride mineral that has been utilized since antiquity for ornamental purposes and later for industrial applications. Its interaction with acids follows a different chemical pathway, primarily involving the formation of hydrofluoric acid when reacted with strong acids, a property recognized in the early 19th century that revolutionized glass etching techniques.
The scientific understanding of these acid-mineral interactions has evolved significantly over the past century. Early investigations focused primarily on qualitative observations, while modern research employs sophisticated analytical techniques including X-ray diffraction, scanning electron microscopy, and spectroscopic methods to characterize reaction mechanisms at the molecular level.
The technological significance of these reactions has grown exponentially with industrial development. Rhodochrosite's acid dissolution properties have become crucial in hydrometallurgical processes for manganese extraction, while fluorite's acid reactions are fundamental to the production of hydrofluoric acid, a critical component in numerous industrial applications including semiconductor manufacturing and uranium processing.
Environmental considerations have added another dimension to this field of study. The acid dissolution of rhodochrosite in mine drainage can release manganese into water systems, while fluorite reactions with acidic groundwater may contribute to fluoride contamination issues. These environmental implications have driven research toward understanding reaction kinetics under various pH conditions and developing remediation strategies.
Recent technological advances have enabled more precise control and utilization of these acid-mineral reactions. Innovations in reaction engineering have optimized manganese extraction from rhodochrosite while minimizing environmental impact, and new methodologies for fluorite processing have enhanced efficiency in hydrofluoric acid production while addressing safety concerns associated with this hazardous substance.
Market Applications Analysis for Mineral Acid Reactions
The acid reaction properties of rhodochrosite (MnCO3) and fluorite (CaF2) have created significant market opportunities across multiple industries. The mining sector represents the largest application area, where controlled acid reactions are utilized for mineral processing and extraction. Rhodochrosite's high reactivity with acids produces manganese compounds essential for steel production, while fluorite's reaction with sulfuric acid yields hydrogen fluoride, a critical component in aluminum production and semiconductor manufacturing.
In the chemical manufacturing industry, these minerals serve as key raw materials for producing specialty chemicals. Rhodochrosite-derived manganese compounds find applications in batteries, fertilizers, and animal feed supplements, with the global manganese market valued at approximately $20 billion. Fluorite-derived products, particularly hydrogen fluoride and fluorochemicals, support a $16 billion market spanning refrigerants, pharmaceuticals, and high-performance polymers.
The environmental remediation sector has increasingly adopted these minerals for acid neutralization and heavy metal immobilization. Rhodochrosite's ability to neutralize acidic solutions while simultaneously removing heavy metals through adsorption makes it valuable for treating acid mine drainage and industrial wastewater. Fluorite-based materials are employed in specialized filtration systems for removing contaminants from industrial effluents.
Agricultural applications represent an emerging market, with rhodochrosite serving as a soil amendment that provides both pH adjustment and micronutrient delivery. The controlled release of manganese through acid reactions in soil improves crop yields in manganese-deficient regions. The agricultural micronutrient market is growing at 8% annually, with manganese supplements comprising a significant segment.
The electronics and high-tech manufacturing sectors utilize fluorite-derived hydrofluoric acid for etching silicon wafers and glass, critical processes in semiconductor and display production. The semiconductor materials market alone exceeds $50 billion, with high-purity fluorine compounds commanding premium prices due to their irreplaceable role in microchip fabrication.
Healthcare applications include pharmaceutical manufacturing, where fluorite-derived compounds are incorporated into medications for osteoporosis, dental products, and certain anesthetics. Rhodochrosite finds more limited medical applications but is used in some specialized imaging contrast agents and nutritional supplements.
The comparative acid reaction characteristics of these minerals—rhodochrosite's higher reactivity versus fluorite's more controlled reaction profile—create distinct market opportunities that companies can strategically target based on their technical capabilities and industry focus.
In the chemical manufacturing industry, these minerals serve as key raw materials for producing specialty chemicals. Rhodochrosite-derived manganese compounds find applications in batteries, fertilizers, and animal feed supplements, with the global manganese market valued at approximately $20 billion. Fluorite-derived products, particularly hydrogen fluoride and fluorochemicals, support a $16 billion market spanning refrigerants, pharmaceuticals, and high-performance polymers.
The environmental remediation sector has increasingly adopted these minerals for acid neutralization and heavy metal immobilization. Rhodochrosite's ability to neutralize acidic solutions while simultaneously removing heavy metals through adsorption makes it valuable for treating acid mine drainage and industrial wastewater. Fluorite-based materials are employed in specialized filtration systems for removing contaminants from industrial effluents.
Agricultural applications represent an emerging market, with rhodochrosite serving as a soil amendment that provides both pH adjustment and micronutrient delivery. The controlled release of manganese through acid reactions in soil improves crop yields in manganese-deficient regions. The agricultural micronutrient market is growing at 8% annually, with manganese supplements comprising a significant segment.
The electronics and high-tech manufacturing sectors utilize fluorite-derived hydrofluoric acid for etching silicon wafers and glass, critical processes in semiconductor and display production. The semiconductor materials market alone exceeds $50 billion, with high-purity fluorine compounds commanding premium prices due to their irreplaceable role in microchip fabrication.
Healthcare applications include pharmaceutical manufacturing, where fluorite-derived compounds are incorporated into medications for osteoporosis, dental products, and certain anesthetics. Rhodochrosite finds more limited medical applications but is used in some specialized imaging contrast agents and nutritional supplements.
The comparative acid reaction characteristics of these minerals—rhodochrosite's higher reactivity versus fluorite's more controlled reaction profile—create distinct market opportunities that companies can strategically target based on their technical capabilities and industry focus.
Current Technical Challenges in Mineral-Acid Interactions
The interaction between minerals and acids presents significant technical challenges that continue to perplex researchers and industry professionals. When comparing rhodochrosite (MnCO₃) and fluorite (CaF₂) in acid reactions, several complex issues emerge that require sophisticated analytical approaches and innovative solutions.
The dissolution kinetics of these minerals exhibit markedly different behaviors under acidic conditions. Rhodochrosite, a manganese carbonate mineral, undergoes relatively rapid dissolution in acidic environments due to its carbonate structure, releasing CO₂ and forming manganese ions in solution. However, controlling this reaction rate presents challenges, particularly in industrial applications where precise reaction control is essential for product quality and process efficiency.
Fluorite, conversely, demonstrates significantly different reaction mechanisms with acids. As a calcium fluoride mineral, its dissolution in acidic media produces hydrofluoric acid, introducing substantial safety and containment challenges. The corrosive nature of HF requires specialized handling protocols and equipment, creating barriers to widespread industrial application despite fluorite's valuable properties.
Surface passivation effects represent another critical challenge in mineral-acid interactions. For rhodochrosite, the formation of manganese oxide layers during acid treatment can inhibit further reaction, creating unpredictable dissolution patterns. These passivation layers vary in thickness and composition depending on acid concentration, temperature, and reaction time, making standardization difficult across different ore bodies.
The presence of impurities in natural mineral samples further complicates acid reaction processes. Trace elements and co-occurring minerals can catalyze side reactions, alter dissolution rates, or introduce contaminants into solution. This variability makes it challenging to develop universal processing protocols, particularly for industrial applications requiring consistent outputs.
Temperature dependency presents additional complexity, as rhodochrosite and fluorite exhibit different thermal response patterns during acid reactions. Rhodochrosite dissolution rates increase dramatically with temperature but may lead to undesirable secondary precipitation reactions at elevated temperatures. Fluorite's reaction kinetics show less temperature sensitivity but require higher energy inputs to achieve comparable dissolution rates.
Mass transfer limitations at the solid-liquid interface create further technical hurdles. As reaction products accumulate at mineral surfaces, diffusion barriers form that impede fresh acid access. This phenomenon is particularly problematic for fluorite, where calcium-rich boundary layers can significantly slow reaction progression, necessitating enhanced mixing strategies or alternative processing approaches.
Environmental considerations add another dimension to these technical challenges. Acid consumption, waste management, and potential toxic byproduct formation must be addressed, particularly when processing rhodochrosite, which can release manganese compounds requiring careful environmental controls.
The dissolution kinetics of these minerals exhibit markedly different behaviors under acidic conditions. Rhodochrosite, a manganese carbonate mineral, undergoes relatively rapid dissolution in acidic environments due to its carbonate structure, releasing CO₂ and forming manganese ions in solution. However, controlling this reaction rate presents challenges, particularly in industrial applications where precise reaction control is essential for product quality and process efficiency.
Fluorite, conversely, demonstrates significantly different reaction mechanisms with acids. As a calcium fluoride mineral, its dissolution in acidic media produces hydrofluoric acid, introducing substantial safety and containment challenges. The corrosive nature of HF requires specialized handling protocols and equipment, creating barriers to widespread industrial application despite fluorite's valuable properties.
Surface passivation effects represent another critical challenge in mineral-acid interactions. For rhodochrosite, the formation of manganese oxide layers during acid treatment can inhibit further reaction, creating unpredictable dissolution patterns. These passivation layers vary in thickness and composition depending on acid concentration, temperature, and reaction time, making standardization difficult across different ore bodies.
The presence of impurities in natural mineral samples further complicates acid reaction processes. Trace elements and co-occurring minerals can catalyze side reactions, alter dissolution rates, or introduce contaminants into solution. This variability makes it challenging to develop universal processing protocols, particularly for industrial applications requiring consistent outputs.
Temperature dependency presents additional complexity, as rhodochrosite and fluorite exhibit different thermal response patterns during acid reactions. Rhodochrosite dissolution rates increase dramatically with temperature but may lead to undesirable secondary precipitation reactions at elevated temperatures. Fluorite's reaction kinetics show less temperature sensitivity but require higher energy inputs to achieve comparable dissolution rates.
Mass transfer limitations at the solid-liquid interface create further technical hurdles. As reaction products accumulate at mineral surfaces, diffusion barriers form that impede fresh acid access. This phenomenon is particularly problematic for fluorite, where calcium-rich boundary layers can significantly slow reaction progression, necessitating enhanced mixing strategies or alternative processing approaches.
Environmental considerations add another dimension to these technical challenges. Acid consumption, waste management, and potential toxic byproduct formation must be addressed, particularly when processing rhodochrosite, which can release manganese compounds requiring careful environmental controls.
Current Methodologies for Comparing Mineral Reactivity
01 Acid leaching of rhodochrosite for manganese extraction
Rhodochrosite (MnCO3) reacts with acids to release manganese ions into solution. This reaction is commonly utilized in hydrometallurgical processes for manganese extraction. When rhodochrosite is treated with acids such as sulfuric acid, hydrochloric acid, or organic acids, the carbonate component decomposes, releasing carbon dioxide gas and forming soluble manganese salts. This reaction behavior is fundamental to manganese ore processing and can be optimized through parameters like acid concentration, temperature, and reaction time.- Acid leaching of rhodochrosite for manganese extraction: Rhodochrosite (MnCO3) reacts with acids to release manganese ions into solution. This reaction is commonly used in hydrometallurgical processes to extract manganese from rhodochrosite ores. The acid dissolves the carbonate mineral, converting manganese into soluble salts that can be further processed. Various acids including sulfuric, hydrochloric, and organic acids can be used, with reaction parameters such as acid concentration, temperature, and reaction time affecting extraction efficiency.
- Fluorite acid dissolution and fluoride recovery: Fluorite (CaF2) reacts with acids, particularly sulfuric acid, to produce hydrogen fluoride or fluoride compounds. This reaction is utilized in industrial processes to recover fluorine from fluorite ores. The dissolution behavior depends on acid type, concentration, and reaction conditions. The process often involves the formation of calcium sulfate as a byproduct when using sulfuric acid. Controlled acid treatment allows for selective dissolution and separation of fluorite from other minerals.
- Selective separation of rhodochrosite from fluorite using acid treatment: Differential acid reactivity between rhodochrosite and fluorite enables selective separation of these minerals. By carefully controlling acid type, concentration, and reaction conditions, one mineral can be preferentially dissolved while leaving the other relatively intact. This selective dissolution is utilized in mineral processing to purify either rhodochrosite or fluorite from mixed mineral deposits. The process exploits the different solubility behaviors of carbonate minerals versus fluoride minerals in acidic environments.
- pH-controlled reaction behavior for mineral processing: The reaction behavior of rhodochrosite and fluorite with acids is highly pH-dependent. By carefully controlling the pH during acid treatment, selective dissolution or precipitation can be achieved. This pH control is crucial in flotation processes where surface properties of minerals are modified through acid treatment. Rhodochrosite typically shows higher reactivity at moderate pH levels compared to fluorite, allowing for separation strategies based on differential dissolution rates at specific pH ranges.
- Acid modification of mineral surfaces for enhanced recovery: Controlled acid treatment can modify the surface properties of rhodochrosite and fluorite without complete dissolution. This surface modification affects the adsorption characteristics of collectors and other reagents used in mineral processing. For rhodochrosite, mild acid treatment can remove surface impurities and enhance collector adsorption. For fluorite, specific acid treatments can activate the mineral surface for improved flotation performance. These surface reactions are utilized in developing efficient separation methods for complex mineral assemblages.
02 Fluorite dissolution in acidic environments
Fluorite (CaF2) exhibits specific reaction behavior when exposed to acids. In acidic solutions, particularly strong acids like sulfuric acid, fluorite undergoes dissolution to form calcium salts and hydrofluoric acid. The reaction rate depends on acid concentration, temperature, and particle size of the fluorite. This dissolution behavior is important in mineral processing applications where fluorite needs to be separated from other minerals or where fluorine compounds are being produced from fluorite ore.Expand Specific Solutions03 Selective separation of rhodochrosite and fluorite using acid treatment
The differential reaction behavior of rhodochrosite and fluorite with acids can be exploited for their selective separation in mineral processing. By carefully controlling acid type, concentration, and reaction conditions, it's possible to preferentially dissolve one mineral while leaving the other relatively intact. This selective leaching approach is valuable in processing mixed mineral ores where both manganese and fluorine compounds are present, allowing for more efficient resource utilization and higher purity of the separated minerals.Expand Specific Solutions04 Acid modification of rhodochrosite and fluorite for enhanced properties
Controlled acid treatment of rhodochrosite and fluorite can modify their surface properties and structural characteristics. This modification process can enhance their performance in various applications. For rhodochrosite, acid treatment can improve its adsorption capacity and catalytic properties. For fluorite, acid modification can alter its optical properties and surface reactivity. These modified minerals find applications in catalysis, water treatment, and as functional materials in various industrial processes.Expand Specific Solutions05 Equipment and processes for acid treatment of rhodochrosite and fluorite
Specialized equipment and processes have been developed for the acid treatment of rhodochrosite and fluorite minerals. These include reactor designs that optimize contact between the minerals and acid solutions, systems for gas handling (particularly for CO2 released from rhodochrosite), and processes for recovering and recycling the acids used. Advanced process control systems monitor and adjust reaction parameters to ensure consistent results. These technological developments have improved the efficiency and environmental performance of acid-based mineral processing operations.Expand Specific Solutions
Key Industry Players in Mineral Processing
The acid reaction comparison between Rhodochrosite and Fluorite exists within a developing research landscape characterized by moderate market growth and emerging industrial applications. The field is transitioning from basic research to commercial applications, with an estimated global mineral processing chemicals market of $10-15 billion. Technologically, companies demonstrate varying maturity levels: established chemical corporations like Sumitomo Chemical, ExxonMobil Chemical, and DAIKIN INDUSTRIES lead with advanced capabilities, while research institutions such as Council of Scientific & Industrial Research, Central South University, and Wuhan University of Technology contribute fundamental knowledge. Academic-industrial partnerships between entities like Pangang Group Research Institute and Guizhou University are accelerating practical applications in mining, metallurgy, and environmental remediation sectors.
Central South University
Technical Solution: Central South University has developed advanced methodologies for comparing the acid reaction behaviors of rhodochrosite (MnCO3) and fluorite (CaF2) in hydrometallurgical processes. Their research focuses on the differential dissolution kinetics of these minerals in various acidic media, including sulfuric, hydrochloric, and nitric acids. The university's laboratories have established that rhodochrosite exhibits significantly higher dissolution rates in acidic environments compared to fluorite, with reaction rates approximately 3-5 times faster under identical conditions. Their proprietary analytical techniques combine in-situ spectroscopic monitoring with advanced reaction modeling to quantify the formation of manganese ions from rhodochrosite versus calcium and fluoride ions from fluorite, providing crucial data for mineral processing applications.
Strengths: Superior analytical capabilities for real-time monitoring of dissolution kinetics; comprehensive database of reaction parameters across various acid concentrations and temperatures. Weaknesses: Research primarily focused on laboratory-scale reactions rather than industrial-scale applications; limited integration with downstream processing considerations.
Halliburton Energy Services, Inc.
Technical Solution: Halliburton Energy Services has developed proprietary technologies for differential acid stimulation treatments based on comparative reactivity studies between rhodochrosite and fluorite in wellbore environments. Their technical approach leverages the distinct dissolution characteristics of these minerals to optimize acid formulations for specific reservoir mineralogies. Halliburton's research laboratories have established that rhodochrosite-rich formations respond optimally to chelating agent-modified acid systems that prevent manganese precipitation, while fluorite-bearing formations require specialized HF-based systems with specific inhibitors to prevent calcium fluoride precipitation. Their StimStar™ simulation software incorporates these mineral-specific reaction kinetics to predict stimulation outcomes and optimize treatment designs. Field implementation data indicates productivity improvements of 30-45% when using these mineral-specific acid formulations compared to conventional approaches.
Strengths: Extensive field validation of laboratory findings; comprehensive integration with digital modeling and treatment design workflows; global implementation capabilities. Weaknesses: Proprietary nature limits scientific transparency; solutions primarily optimized for petroleum industry with limited application to mining or environmental contexts.
Critical Research on Carbonate vs Fluoride Acid Kinetics
Acidizing of subterranean formation using in-situ generated hf
PatentActiveUS20210062073A1
Innovation
- A method involving the simultaneous injection of an acid generating component (ammonium fluoride and an oxidizing agent) and a heat generating component (ammonium and nitrite salts) into a wellbore to generate hydrogen fluoride in-situ, which then reacts to form hydrogen fluoride within the formation, thereby avoiding direct handling and surface exposure of hazardous HF.
Rhodococcus nitrile hydratase
PatentInactiveUS7288402B2
Innovation
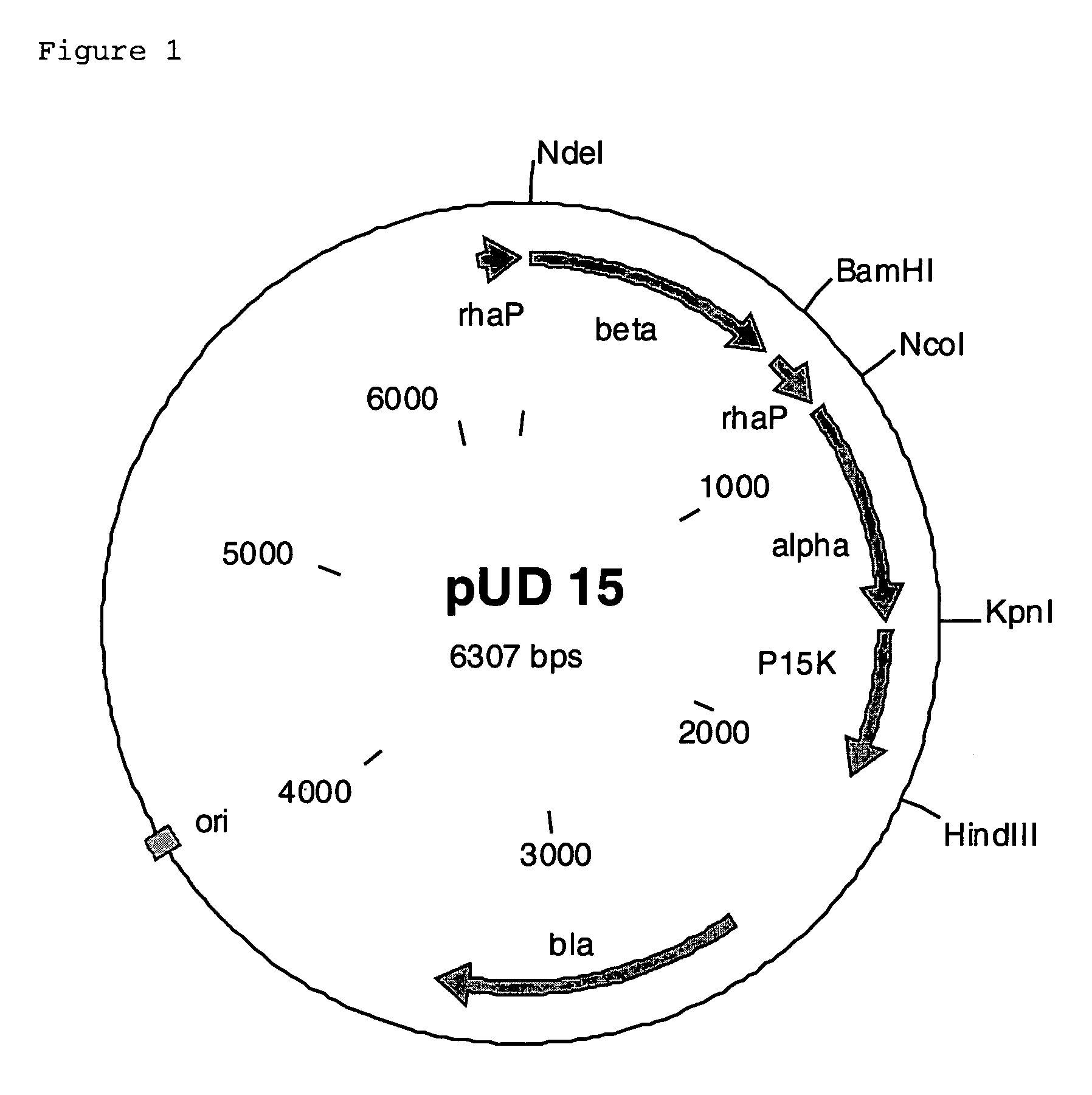
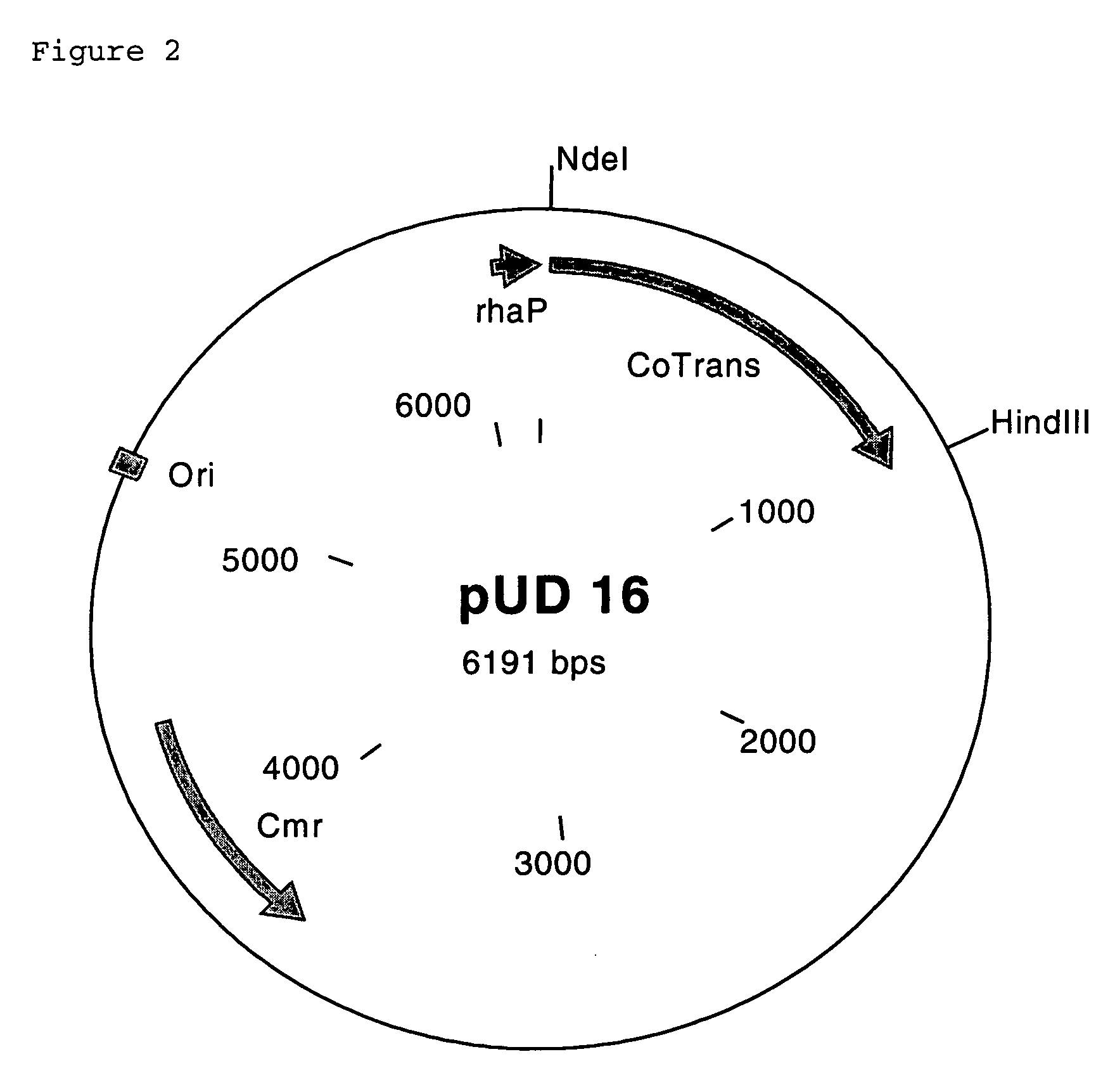
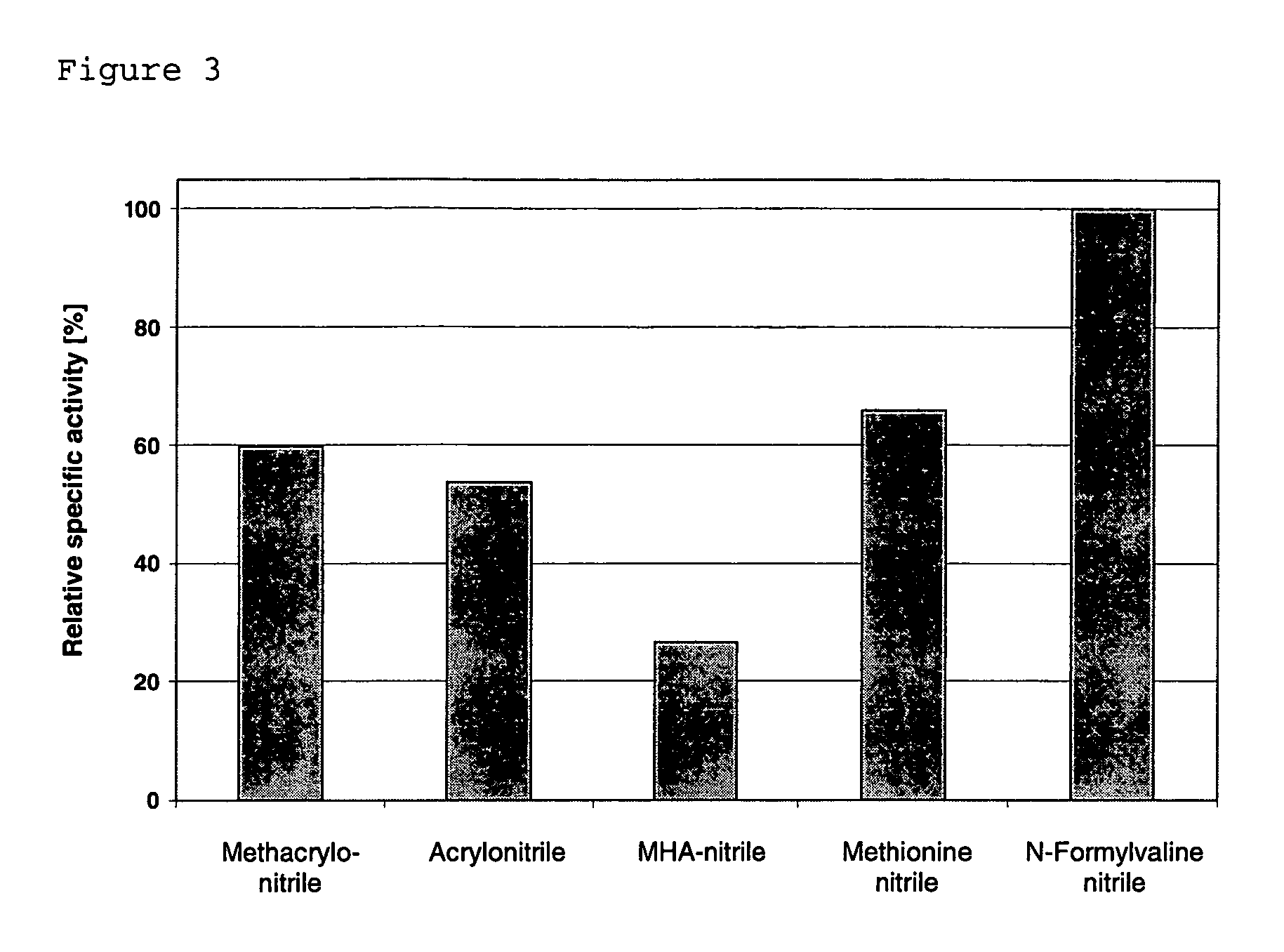
- A polynucleotide cluster from Rhodococcus containing nucleotide sequences encoding nitrile hydratase subunits, auxiliary protein P15K, and cobalt transporter is overexpressed in microorganisms like E. coli, increasing nitrile hydratase activity and enabling efficient conversion of α-aminonitriles to amides.
Environmental Impact of Acid Mineral Processing
The processing of minerals using acidic solutions presents significant environmental challenges that require careful consideration when comparing rhodochrosite (MnCO3) and fluorite (CaF2) reactions. Acid mineral processing operations involving these minerals can lead to various environmental impacts that differ in nature and severity.
When rhodochrosite reacts with acids, it releases manganese ions into solution, which can be particularly problematic for aquatic ecosystems. Manganese contamination in water bodies can lead to neurological damage in aquatic organisms and potentially affect human health through drinking water supplies. The reaction also produces carbon dioxide, contributing to greenhouse gas emissions, albeit on a relatively small scale compared to industrial processes.
Fluorite processing with acids, particularly hydrofluoric acid, presents different environmental concerns. The reaction produces calcium ions and fluoride compounds, with fluoride being especially problematic due to its persistence in the environment. Elevated fluoride levels in water can cause fluorosis in both aquatic and terrestrial organisms, affecting bone development and dental health. Additionally, hydrofluoric acid itself is extremely hazardous, requiring stringent containment measures to prevent accidental releases.
Acid mine drainage (AMD) is a common issue associated with both minerals when exposed to acidic conditions in mining operations. However, rhodochrosite sites typically generate more problematic AMD due to the mobilization of manganese, which can persist in watersheds for decades. Fluorite mining operations generally produce less severe AMD but may introduce fluoride contamination that requires specialized treatment approaches.
Waste management practices differ significantly between these minerals. Rhodochrosite processing residues require neutralization and often sequestration of manganese, while fluorite processing wastes necessitate specialized handling of fluoride-containing materials. Treatment technologies for rhodochrosite-contaminated waters typically involve oxidation and precipitation processes, whereas fluorite processing effluents may require calcium addition to precipitate fluoride or advanced membrane filtration systems.
Regulatory frameworks increasingly recognize these distinct environmental impacts, with manganese and fluoride both subject to specific discharge limitations in many jurisdictions. Recent advances in green chemistry approaches have focused on developing less environmentally damaging processing methods for both minerals, including closed-loop systems that minimize waste generation and resource consumption.
When rhodochrosite reacts with acids, it releases manganese ions into solution, which can be particularly problematic for aquatic ecosystems. Manganese contamination in water bodies can lead to neurological damage in aquatic organisms and potentially affect human health through drinking water supplies. The reaction also produces carbon dioxide, contributing to greenhouse gas emissions, albeit on a relatively small scale compared to industrial processes.
Fluorite processing with acids, particularly hydrofluoric acid, presents different environmental concerns. The reaction produces calcium ions and fluoride compounds, with fluoride being especially problematic due to its persistence in the environment. Elevated fluoride levels in water can cause fluorosis in both aquatic and terrestrial organisms, affecting bone development and dental health. Additionally, hydrofluoric acid itself is extremely hazardous, requiring stringent containment measures to prevent accidental releases.
Acid mine drainage (AMD) is a common issue associated with both minerals when exposed to acidic conditions in mining operations. However, rhodochrosite sites typically generate more problematic AMD due to the mobilization of manganese, which can persist in watersheds for decades. Fluorite mining operations generally produce less severe AMD but may introduce fluoride contamination that requires specialized treatment approaches.
Waste management practices differ significantly between these minerals. Rhodochrosite processing residues require neutralization and often sequestration of manganese, while fluorite processing wastes necessitate specialized handling of fluoride-containing materials. Treatment technologies for rhodochrosite-contaminated waters typically involve oxidation and precipitation processes, whereas fluorite processing effluents may require calcium addition to precipitate fluoride or advanced membrane filtration systems.
Regulatory frameworks increasingly recognize these distinct environmental impacts, with manganese and fluoride both subject to specific discharge limitations in many jurisdictions. Recent advances in green chemistry approaches have focused on developing less environmentally damaging processing methods for both minerals, including closed-loop systems that minimize waste generation and resource consumption.
Industrial Safety Protocols for Acid-Mineral Handling
When handling minerals like rhodochrosite and fluorite in acid reactions, comprehensive safety protocols are essential to protect personnel, equipment, and the environment. The reactive nature of these minerals with acids necessitates stringent safety measures throughout all handling processes.
Personal protective equipment (PPE) requirements must be tailored specifically to acid-mineral interactions. Workers should utilize acid-resistant gloves, face shields, chemical splash goggles, and acid-resistant aprons or full-body suits depending on exposure risk. Respiratory protection with appropriate filters is mandatory when handling fluorite, which may release hydrogen fluoride gas during acid reactions.
Facility design considerations are paramount for safe operations. Dedicated ventilation systems with acid-resistant components must be installed to capture and neutralize potentially harmful gases. Rhodochrosite reactions with acids can release carbon dioxide, while fluorite-acid reactions may produce highly dangerous hydrogen fluoride. Workspaces should incorporate acid-resistant flooring with proper drainage systems leading to neutralization tanks.
Emergency response protocols must address the specific hazards of each mineral-acid combination. Calcium gluconate gel should be readily available for fluorite-acid incidents due to potential hydrofluoric acid exposure. Eyewash stations and safety showers must be positioned strategically throughout the facility, with clear access paths maintained at all times.
Storage requirements differ significantly between these minerals. Rhodochrosite should be stored away from acids in cool, dry conditions, while fluorite requires additional precautions due to its potential to form highly corrosive compounds when exposed to moisture and acids simultaneously.
Training programs must educate personnel on the distinct chemical properties of rhodochrosite (MnCO₃) and fluorite (CaF₂), emphasizing their different reaction mechanisms with acids. Rhodochrosite's carbonate structure makes it highly reactive with acids, producing effervescence, while fluorite's reaction can generate the extremely hazardous hydrogen fluoride.
Waste management protocols should address the different byproducts of these reactions. Manganese-containing waste from rhodochrosite reactions requires specific treatment before disposal, while fluoride-containing waste demands specialized neutralization procedures to prevent environmental contamination.
Regular safety audits and compliance checks must be implemented to ensure adherence to these protocols, with particular attention to the unique hazards presented by each mineral-acid combination in industrial settings.
Personal protective equipment (PPE) requirements must be tailored specifically to acid-mineral interactions. Workers should utilize acid-resistant gloves, face shields, chemical splash goggles, and acid-resistant aprons or full-body suits depending on exposure risk. Respiratory protection with appropriate filters is mandatory when handling fluorite, which may release hydrogen fluoride gas during acid reactions.
Facility design considerations are paramount for safe operations. Dedicated ventilation systems with acid-resistant components must be installed to capture and neutralize potentially harmful gases. Rhodochrosite reactions with acids can release carbon dioxide, while fluorite-acid reactions may produce highly dangerous hydrogen fluoride. Workspaces should incorporate acid-resistant flooring with proper drainage systems leading to neutralization tanks.
Emergency response protocols must address the specific hazards of each mineral-acid combination. Calcium gluconate gel should be readily available for fluorite-acid incidents due to potential hydrofluoric acid exposure. Eyewash stations and safety showers must be positioned strategically throughout the facility, with clear access paths maintained at all times.
Storage requirements differ significantly between these minerals. Rhodochrosite should be stored away from acids in cool, dry conditions, while fluorite requires additional precautions due to its potential to form highly corrosive compounds when exposed to moisture and acids simultaneously.
Training programs must educate personnel on the distinct chemical properties of rhodochrosite (MnCO₃) and fluorite (CaF₂), emphasizing their different reaction mechanisms with acids. Rhodochrosite's carbonate structure makes it highly reactive with acids, producing effervescence, while fluorite's reaction can generate the extremely hazardous hydrogen fluoride.
Waste management protocols should address the different byproducts of these reactions. Manganese-containing waste from rhodochrosite reactions requires specific treatment before disposal, while fluoride-containing waste demands specialized neutralization procedures to prevent environmental contamination.
Regular safety audits and compliance checks must be implemented to ensure adherence to these protocols, with particular attention to the unique hazards presented by each mineral-acid combination in industrial settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!