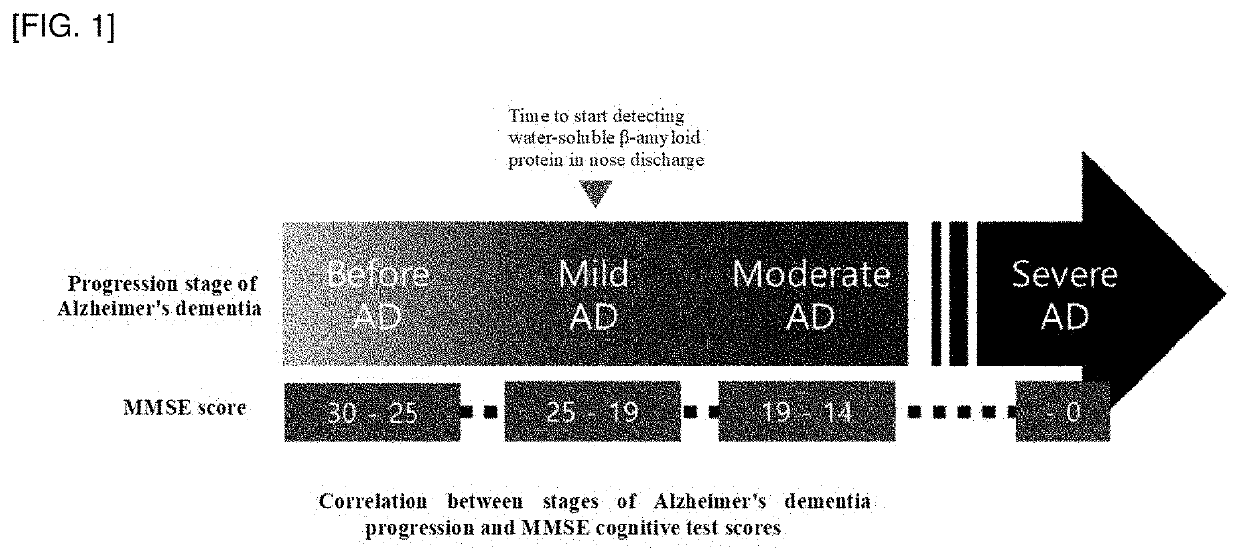
Composition for screening ongoing progress of alzheimer's disease by using beta amyloid oligomer in nasal discharge specimen and method for screening ongoing progress of alzheimer's disease by using same
a technology of beta amyloid and alzheimer's disease, which is applied in the direction of material analysis, biological material analysis, instruments, etc., can solve the problems of high patient cost, time-consuming and costly methods, and abnormal increase or decrease of electrical activity of the brain nerve, so as to screen accurately and quickly, easily and accurately screen alzheimer's onset and stage of development, and save patient cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
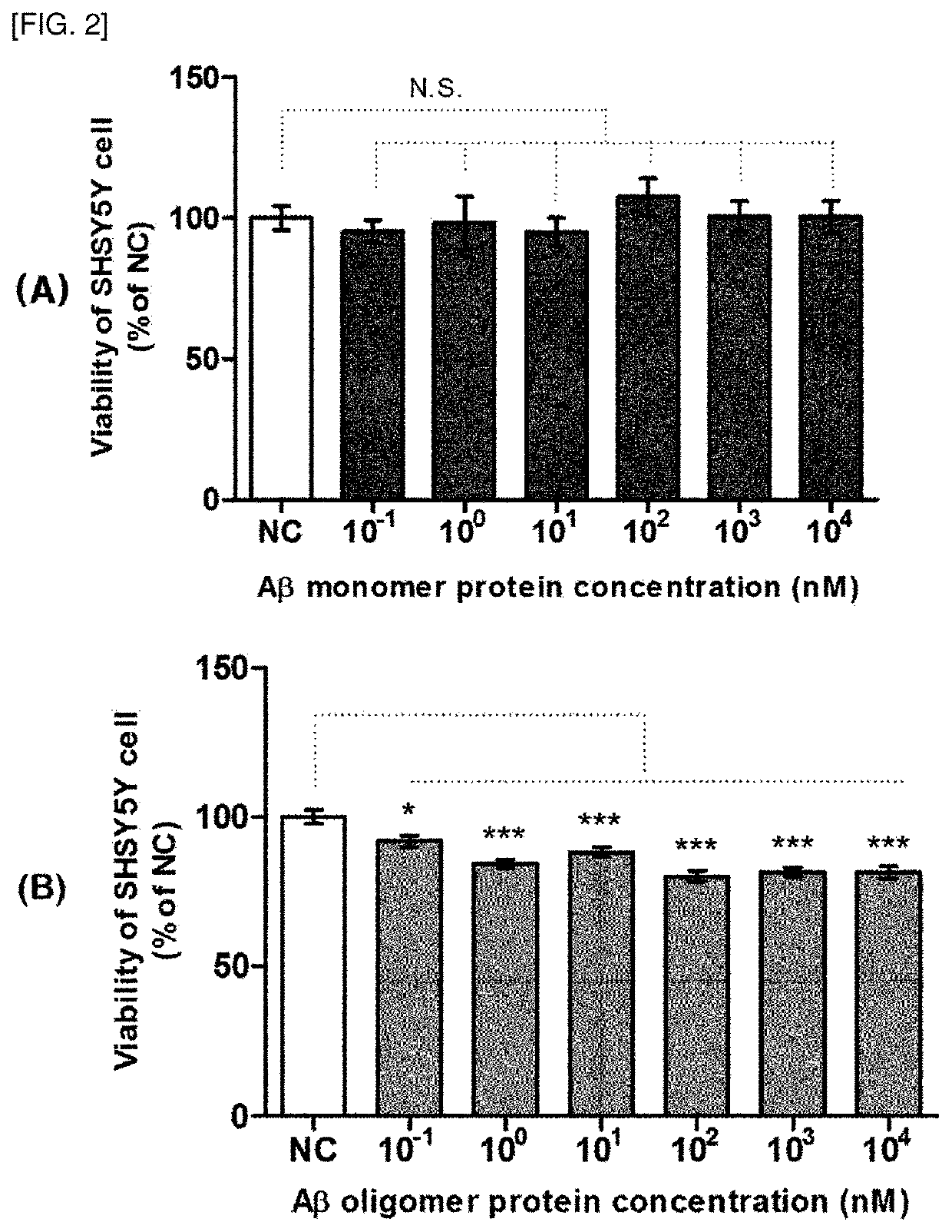
[0133]Toxicity test of beta amyloid monomer and beta amyloid oligomer
[0134]1-1. Preparation of Aβ42 Oligomer
[0135]Aβ42 oligomers were prepared according to the reference (Stine WB et al. Preparing synthetic Abeta in different aggregation states. Methods Mol Biol 2011; 670: 13-32). Briefly, Aβ42 peptide (GL biochem, Shanghai) was initially dissolved in hexafluoroisopropanol at a concentration of 1 mM. For aggregation, the peptide was resuspended in dry dimethylsulfoxide at 5 mM, and then added to Hams F-12 cell culture medium (PromoCell, Labclinics, Spain) at a final concentration of 100 mM for 24 hours at 4° C. In the case of a monomer, the above polymerization process was not performed.
[0136]1-2. Cell Viability Assay
[0137]In order to confirm the toxicity of the beta amyloid monomer and the beta amyloid oligomer prepared in Example 1-1, a cell viability assay was performed using human nerve cancer cell SHSY5Y (ATCC®, CRL-2266) as follows.
[0138]Cell viability was measured using Calce...
example 2
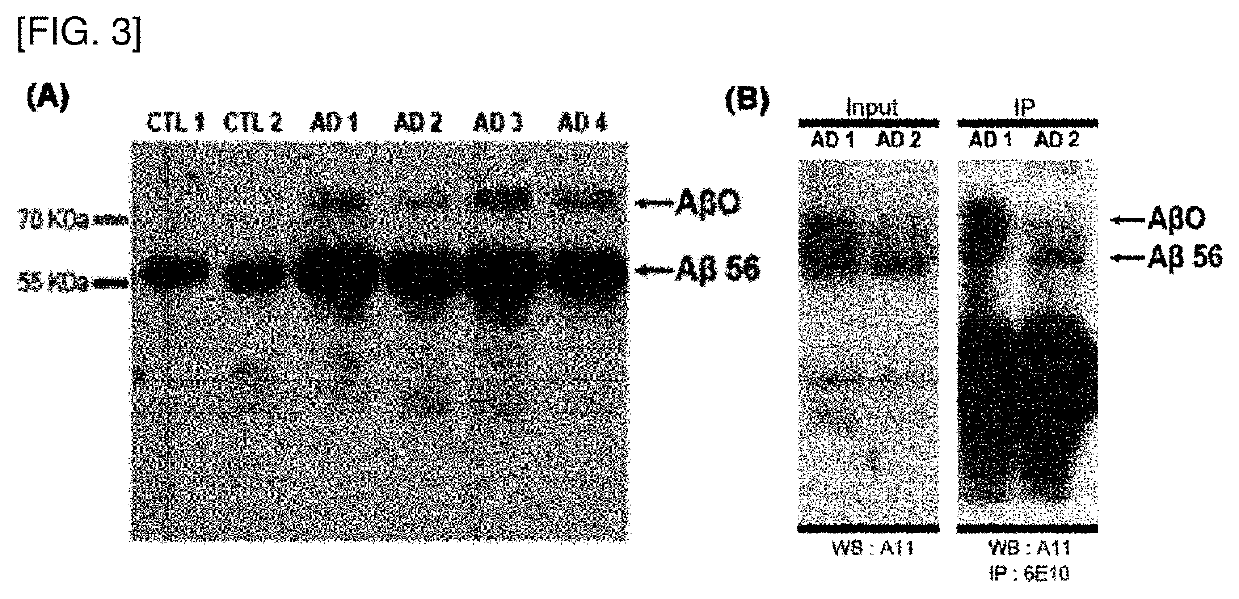
[0139]Identification of Oligomeric Beta Amyloid Protein in Nose Discharge Sample
[0140]2-1. Subject Selection
[0141]The normal control group selected persons over the age of 60 who did not meet the other exclusion criteria and normal cognitive ability on the Seoul Neuropsychological Screening Battery (SNSB), and the patients with Alzheimer's dementia were definite Alzheimer's disease patients according to the NINCDS / ADRDA diagnosis criteria and selected persons with deterioration in two or more cognitive domains, including memory, those who have been identified as having problems with their daily life due to cognitive decline, not other causes through neuropsychological examination, and those who do not meet other exclusion criteria.
[0142]Exclusion criteria include those identified as having no brain structural problems through brain imaging (MRI or CT), people without other degenerative brain diseases, people without cerebrovascular disease that may cause cognitive impairment, people...
example 3
[0153]Quantification of Beta Amyloid Oligomers of 56 kDa and 72 kDa
[0154]As described in the reference [K.-A. Chang et al., Neurochemistry International 97 (2016)], beta amyloid oligomers of 56 kDa and 72 kDa were quantified by classifying Alzheimer's disease patient group and normal group.
[0155]Briefly, the classification criteria for normal control group include age of 65 years or older, a person having K-MMSE score of 25 or higher in the cognitive function test (KMMSE / GDS / CDR) and normal memory item (3 items-3 points) and the classification criteria for moderate Alzheimer's disease include age of 65 years or older and a person having K-MMSE of 19 points or less in the cognitive function test (KMMSE / GDS / CDR), and CDR of 1 point or higher or GDS of 3 points or higher, a person having CDR of 0.5 point and sum of box of 2.5 points or higher, a person who does not have a structural problem in the brain through brain imaging (MRI or CT) tests, a person without other degenerative brain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com