Apparatus and method for promoting angiogenesis in ischemic tissue
a technology which is applied in the field of angiogenesis and angiogenesis in ischemic tissue, can solve the problems of limb/digital gangrene, non-healing ulcers, and rest pain in the foot, and achieve the effect of improving uptake into the surrounding ischemic tissue and reducing washou
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0043]Arteries and veins generally are comprised of three layers: the innermost layer called the intimal layer, the outermost layer called the adventitial layer, and the medial layer located in between the intimal and the adventitial layers. The intimal and medial layers are readily separated from the adventitial layer. For example, it is known that when attempting to pass an occlusion with a guidewire, the guidewire may sometimes inadvertently penetrate the subintimal space between the intimal and the adventitial layer. Hereinafter, in the context of this specification, the term “intimal layer” refers to the intima / media that adjoins the occluded vessel lumen, while the term “adventitial layer” refers to the outer layer of the vessel that may be separated from the intimal layer when a subintimal space is created.
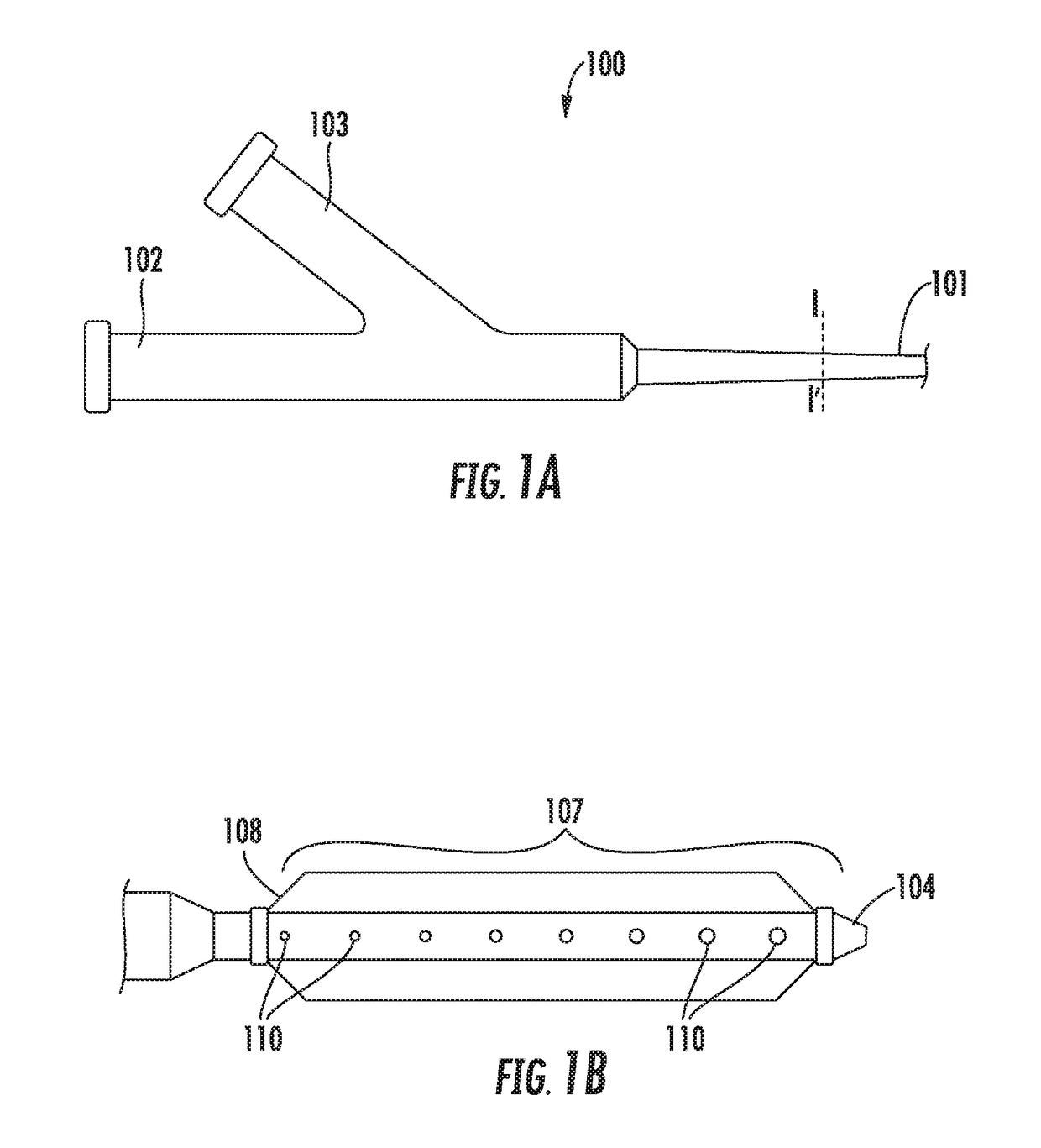
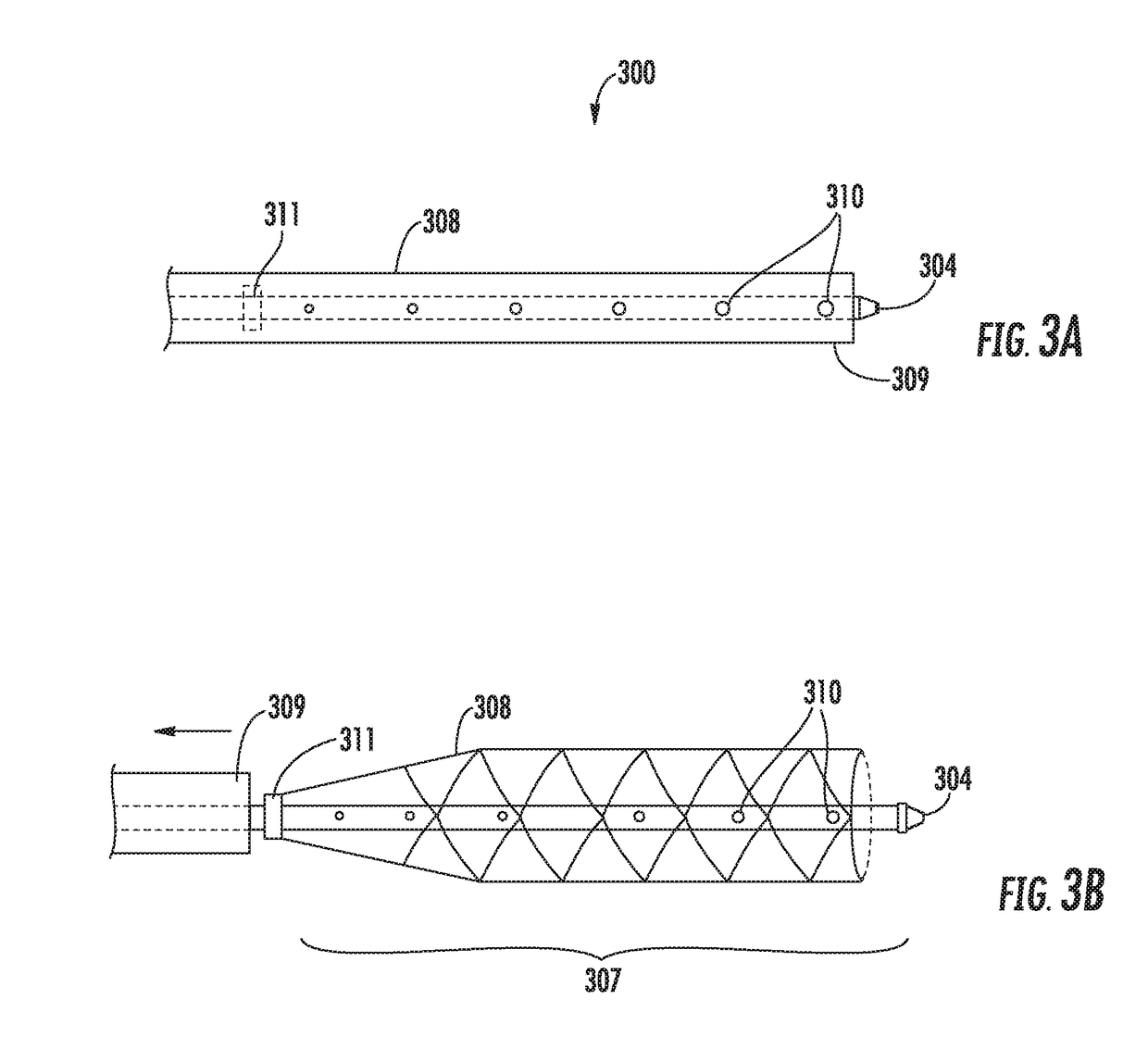
[0044]Referring to FIGS. 1A and 1B, a biologics delivery device constructed in accordance with the principles of the present invention is described. Biologics delivery devi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com