COMPOSITION FOR THE DIAGNOSIS, PREVENTION OR TREATMENT OF DISEASES RELATED TO CELLS EXPRESSING IL-8 OR GRO-ALPHA, COMPRISING UCB-MSCs
a technology of ucbmscs and cells, applied in the direction of drug compositions, diagnostic recording/measuring, ultrasonic/sonic/infrasonic diagnostics, etc., can solve the problems of difficult brain tumor treatment, increased risk of complications, and continuous cell division
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Umbilical Cord Blood-Derived Mesenchymal Stem Cells (UCB-MSCS)
[0227]UCB samples were collected from the umbilical vein of deliveries, with informed maternal consent. Specifically, a 16-gauge needle of a UCB collection bag containing 44 mL of CPDA-1 anticoagulant (Greencross Co., Yongin, Kyungki-do, Korea) was inserted into the umbilical vein and UCB was collected by gravity. In all cases, UCB harvests were processed within 48 hours of collection, with viability of 90% or more.
example 2
Isolation and Expansion of UCB-MSCs
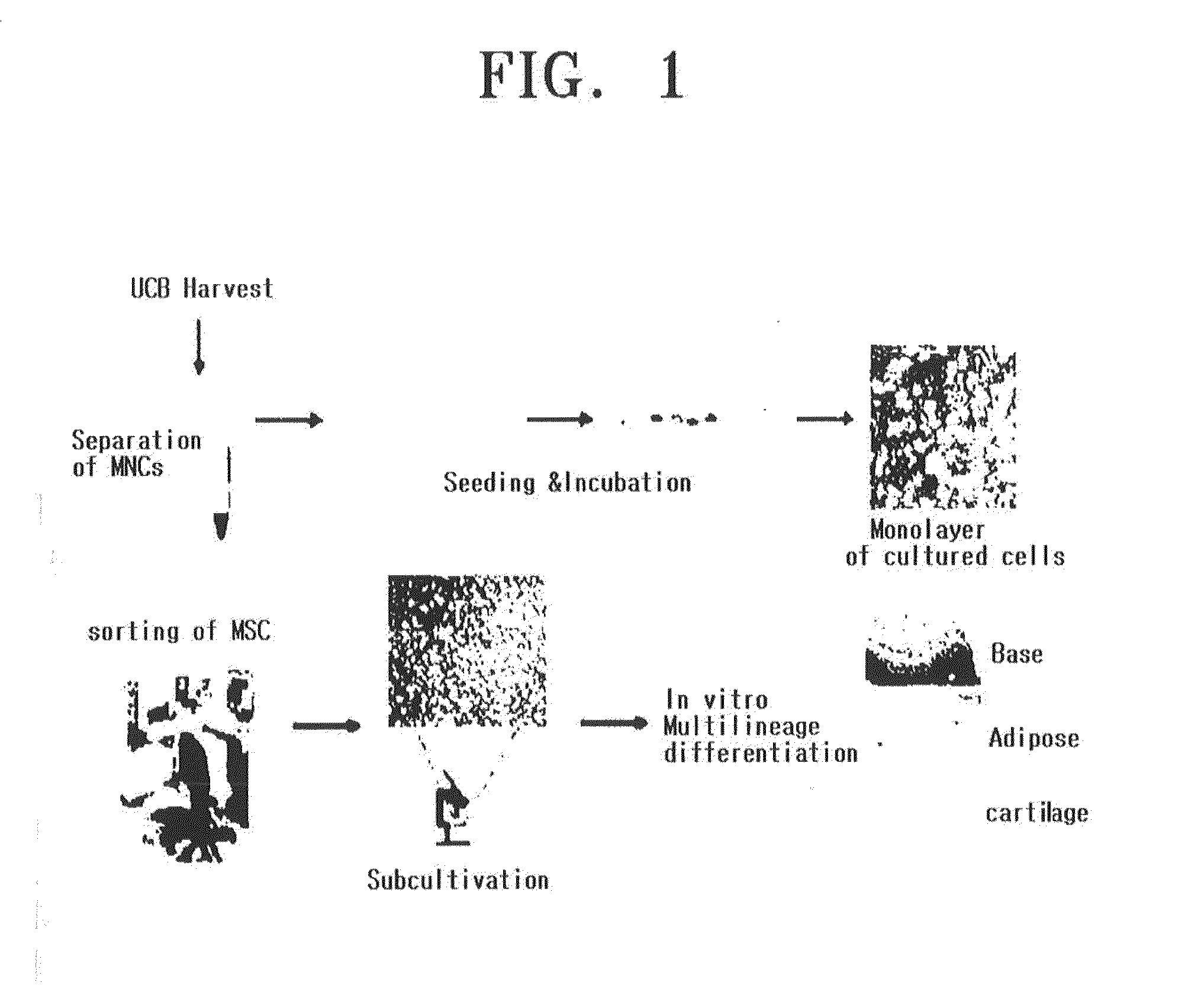
[0228]UCB-MSCs prepared according to Example 1 were centrifuged with a Ficoll-Hypaque gradient (produced by Sigma Co., density: 1.077 g / mL), and then washed several times to to remove impurities. 10 to 20% FBS (HyClone Co.)-containing a basic medium (α-MEM, Gibco BRL Co.) was added to the resultant product to suspend UCB-MSCs. The UCB-MSCs were portioned at a suitable concentration into each of 10 to 20% FBS—containing a basic media, and then cultivated at 37° C. in a 5% CO2 incubator while the medium was altered twice in a week (FIG. 1). When the cultured cells formed a single layer, MSCs expanding in a spindle shape were identified with a phase-contrast microscope. Then, sub-cultivation was repeatedly performed until the MSCs expanded sufficiently.
example 3
Preparation of UCB-MSCs Labeled with PKH-26
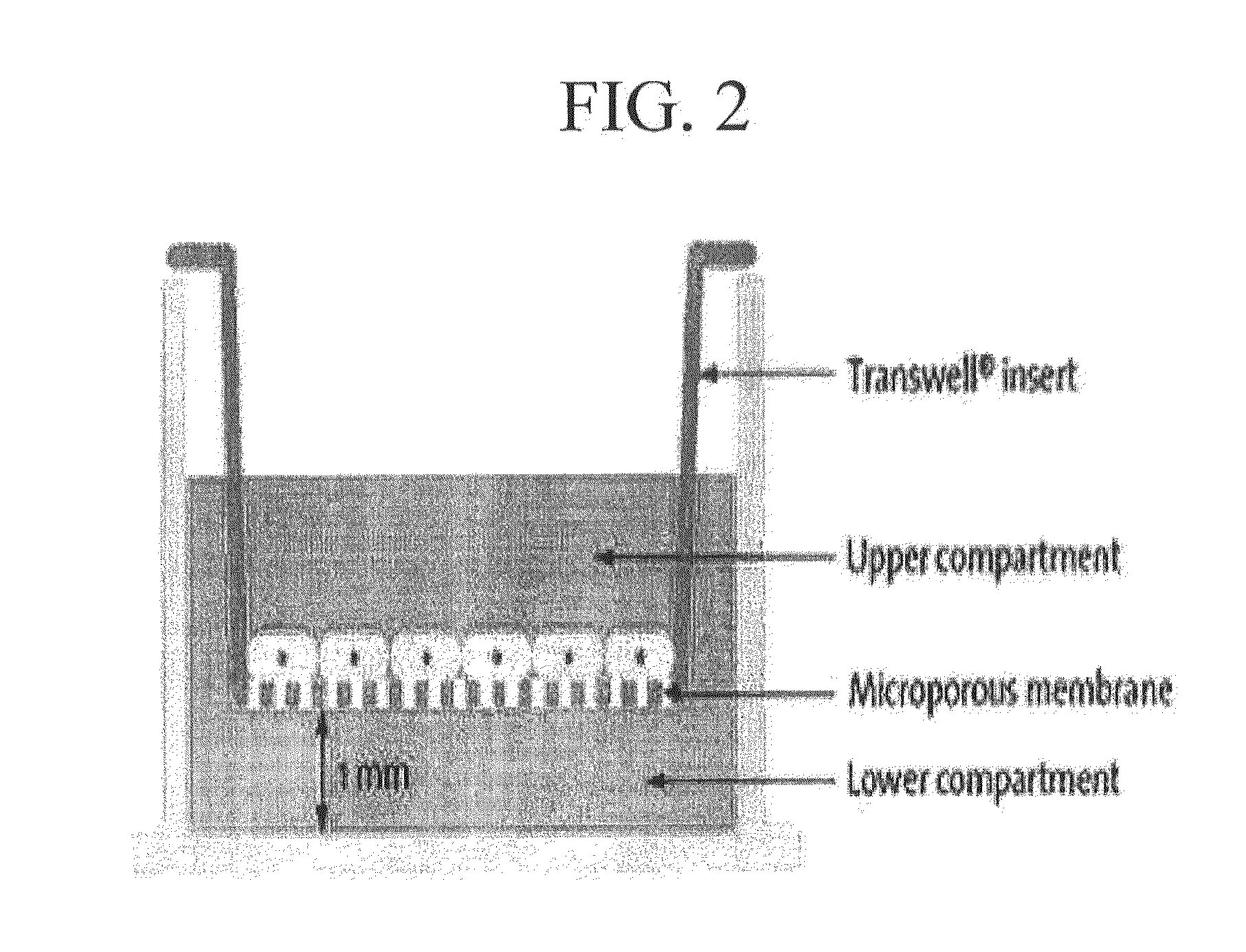
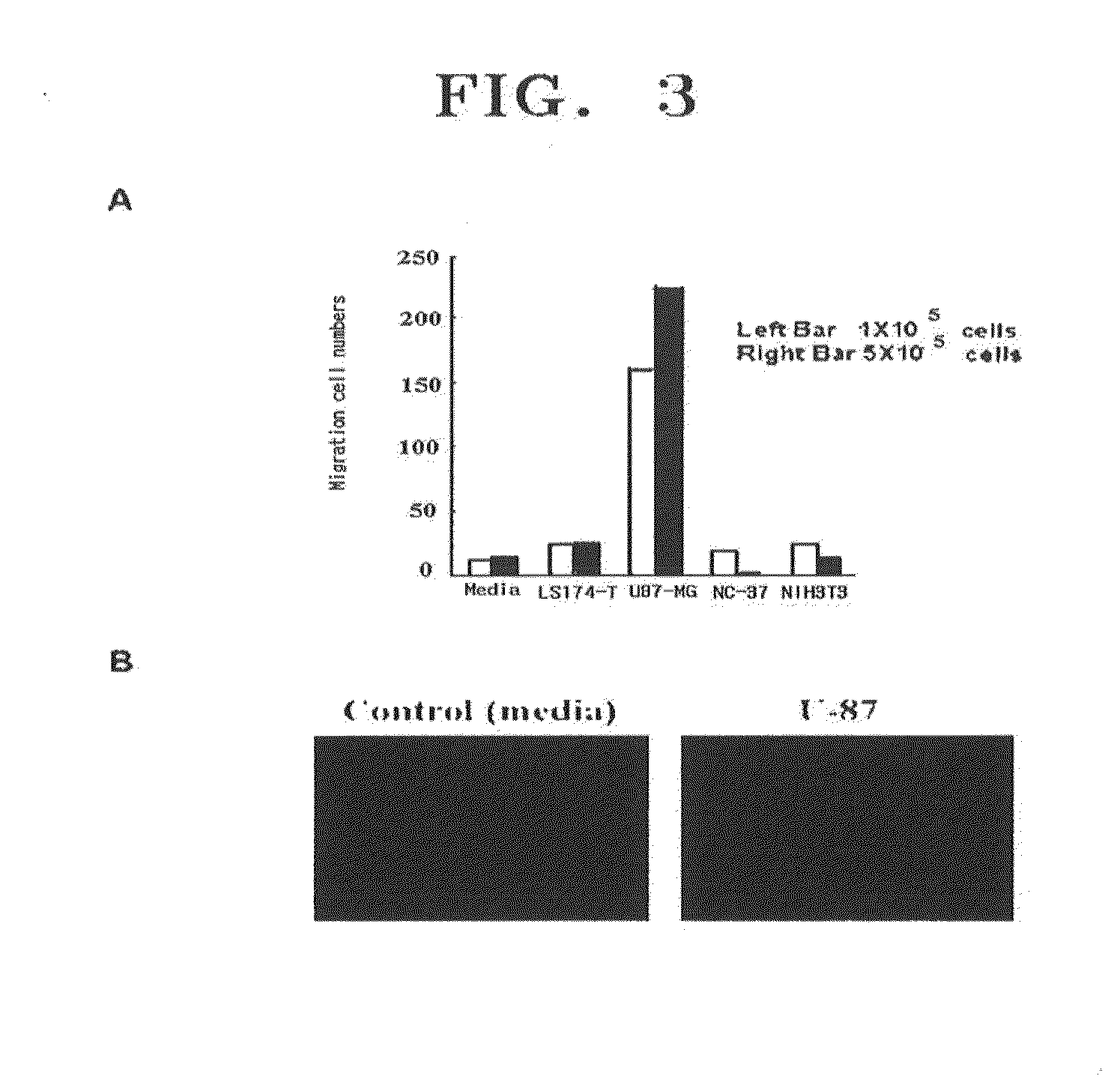
[0229]UCB-MSCs cultivated according to Example 2 were dyed with PKH-26 (Sigma Co.) using a method disclosed in a reference [Barreda D A et al., Developmental and Comparative Immunology, 24:395-406, 2000]. First, UCB-MSCs were separated from the cell culture dish by using Trypsin and then, 2×107 cells were washed with an FBS-free medium. The washed cells were collected using a centrifuge and then suspended in 1 mL of Diluent C in a kit provided by a manufacturer. Then the resultant cell suspension solution (2×) was mixed with 1 ml of the PKH fluorescent dye solution(2×) and then the mixture was reacted at 25° C. for 5 minutes. To terminate the labeling reaction, a medium containing an equal volume of fetal bovine serum (FBS) was added to the reaction product and then left to sit for 1 minute. Cells labeled with PKH26 were collected by centrifuging and then, washed with a 10 to 20% FBS-containing medium three times and used in experiments.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com