Attenuated salmonella enterica serovar paratyphi a and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
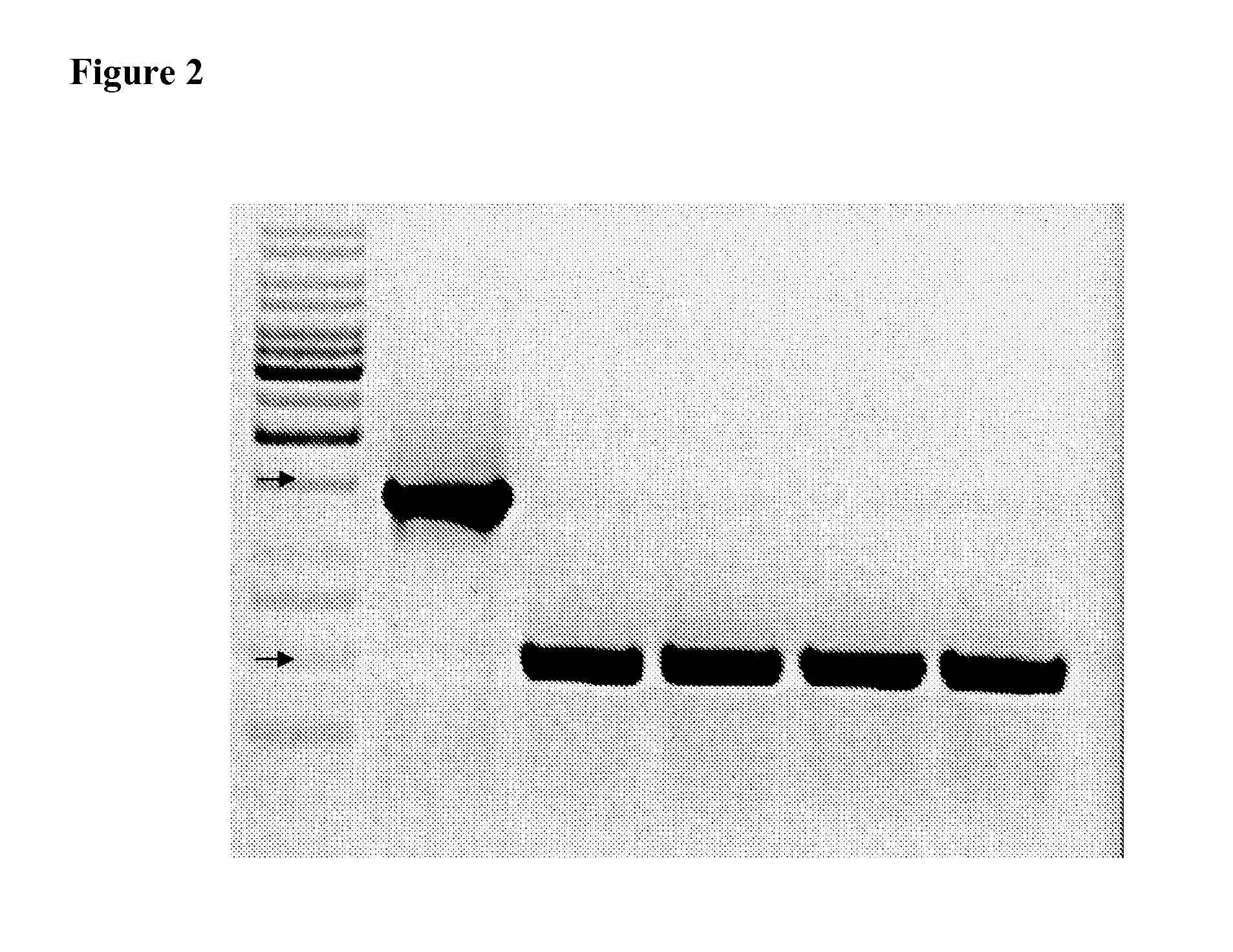
A. Attenuated S. Paratyphi A Strains
[0036]The present invention relates to an attenuated S. Paratyphi A strain. Such attenuated S. Paratyphi A strains may be used to induce an immune response in a subject without causing disease in the subject.
[0037]The S. Paratyphi A strain used as the starting material of the present invention may be any S. Paratyphi A strain and the identity of the strain is not critical. Preferred S. Paratyphi A strains include S. Paratyphi A 9150 strain.
[0038]The S. Paratyphi A strains of the present invention are attenuated. As used herein, attenuated strains of S. Paratyphi A are those that have a reduced, decreased, or suppressed ability to cause disease in a subject, or those completely lacking in the ability to cause disease in a subject. Attenuated strains may exhibit reduced or no expression of one or more genes, may express one or more proteins with reduced or no activity, may exhibit a reduced ability to grow and divide, or a combination of two or more...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com