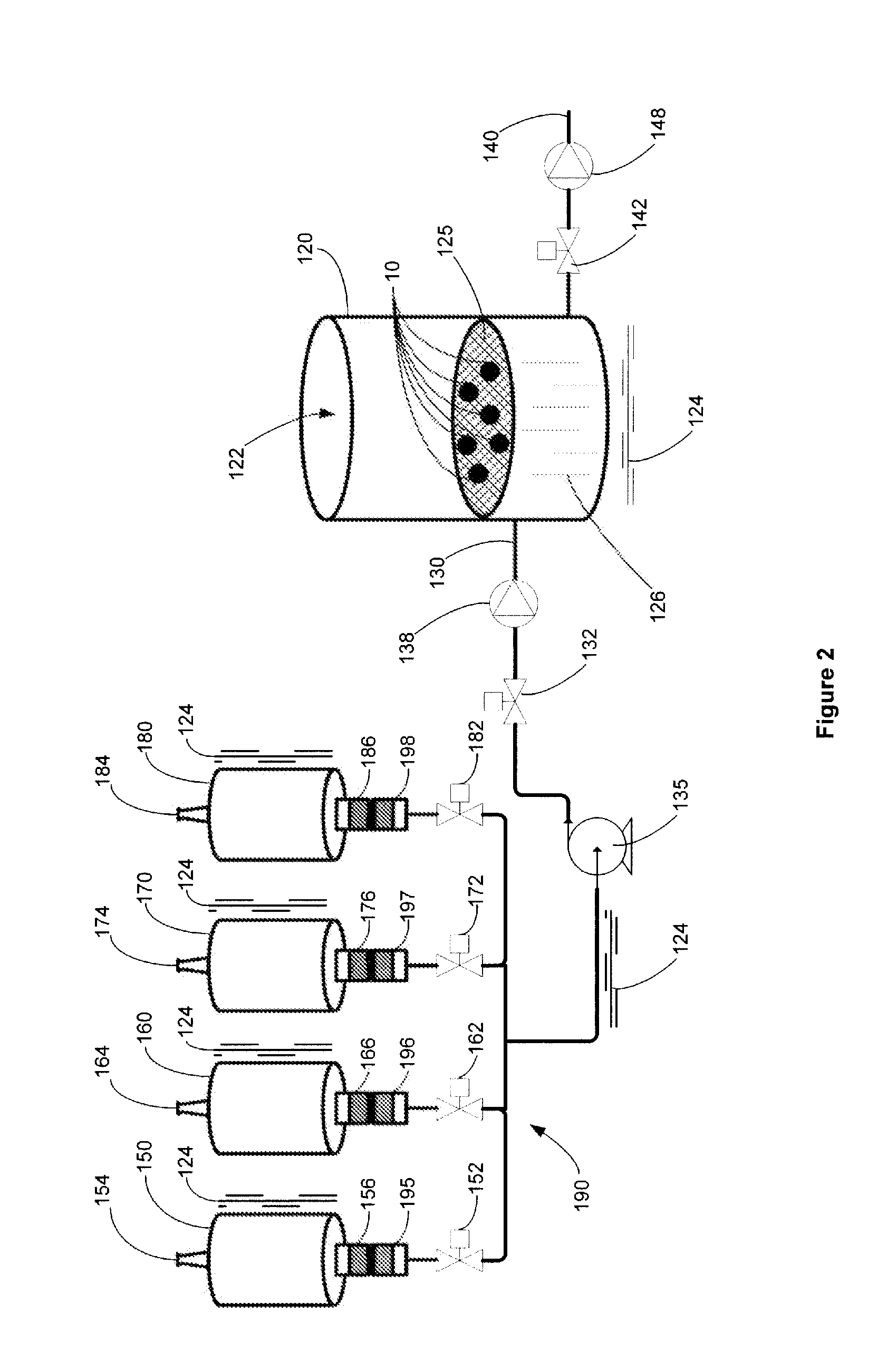
Automated system for cryopreservation of oocytes, embryos, or blastocysts
a technology of cryopreservation and embryo, applied in the field of cryopreservation, can solve the problems of oocytes often suffering structural damage, conventional techniques are typically labor-intensive, and it is difficult to reproduce in an effective, efficient and consistent manner
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026]As used herein, the following definitions shall apply unless otherwise indicated.
[0027]The term “oocyte” refers to an unfertilized freshly harvested or mature oocyte and refers to both the singular and plural regardless of whether this term employs terms such as “a”, “the” and the like. The freshly harvested means that the oocytes were harvested from the animal donor within 8 hours of initiation of the stabilization / cryopreservation process, preferably within about 4 hours of initiation of the stabilization / cryopreservation process, more preferably within about 1 hour of initiation of the stabilization / cryopreservation process, and even more preferably within about 0.1 hour of initiation of the stabilization / cryopreservation process. The mature oocytes mean harvested oocytes which are graded on a maturation scale as “mature stage—MII.” This scale further identifies harvested oocytes as “intermediate stage—(MI)” or “immature stage—(GV)”.
[0028]The term “reanimated oocytes” refer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com