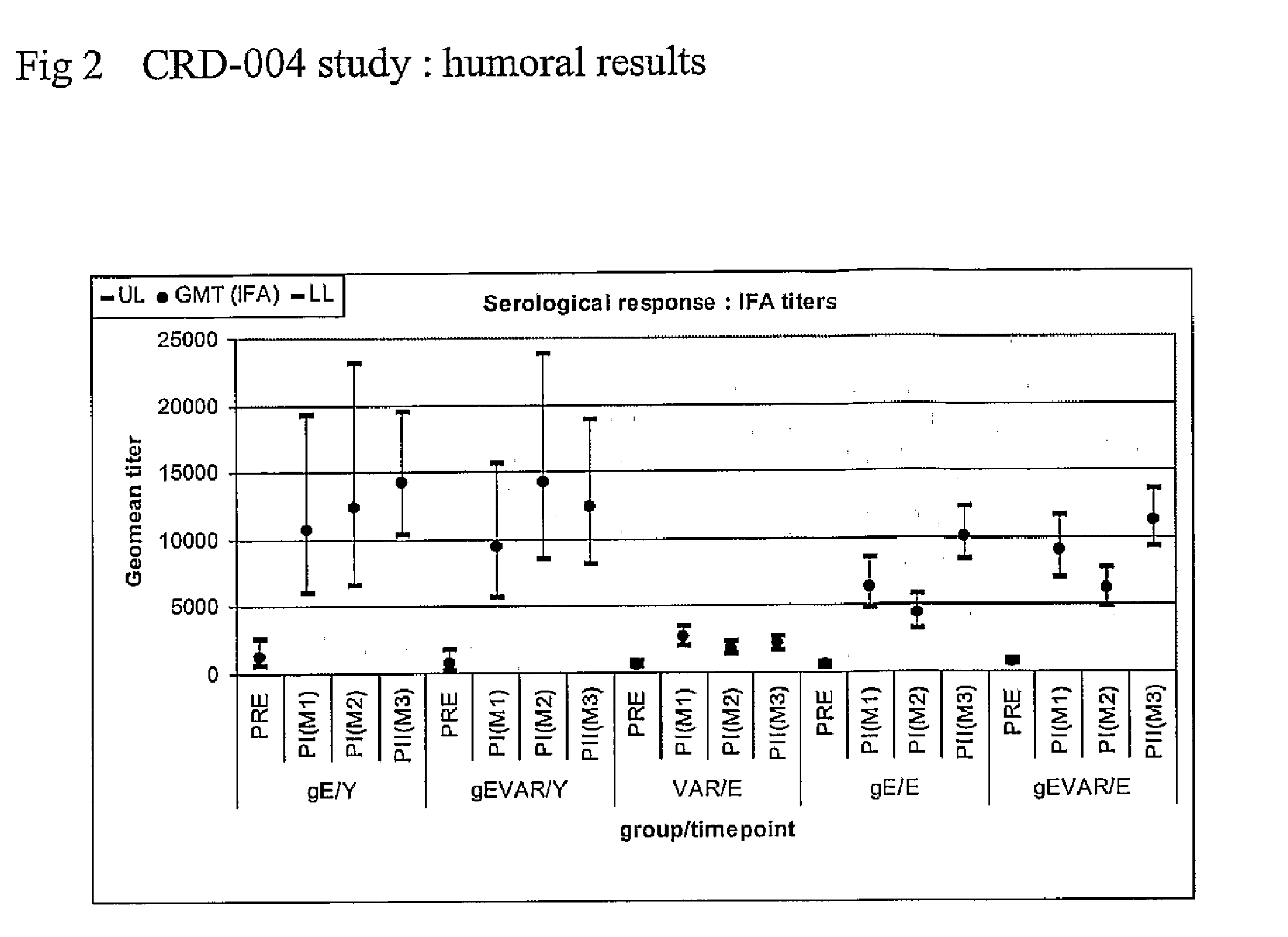
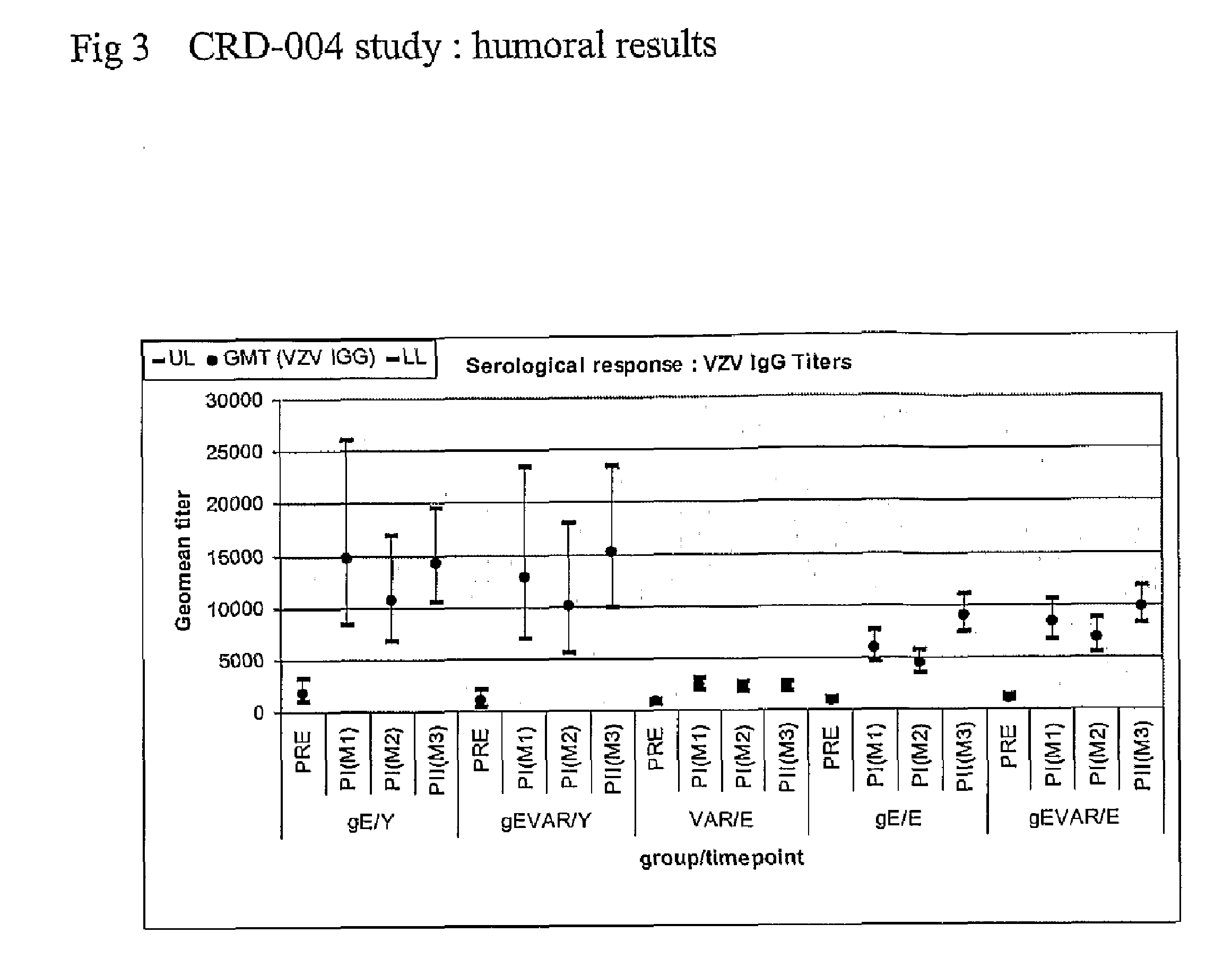
Vaccine against varicella zoster virus
a technology of varicella zoster virus and vaccine, which is applied in the field of vaccine against varicella zoster virus, can solve the problems of life-threatening vzv infection and neonatal vzv infection, and achieve the effect of preventing and/or decreasing the severity of herpes zoster and/or post herpetic neuralgia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0120]Three experimental groups may be set up to study both aspects of the invention:
Regime150 μg gE + adjuvant AS1 (MPL ® / QS21)0, 2 months2OKA strain (Varilrix ™) ~10,000 pfu / dose0, 2 months3Concomitant administration of 50 μg gE + AS1 group0, 2 months(as in 1) with Varilrix ™ (as in 2)MPL ® = 3D-MPL
[0121]The gE used can be truncated gE, as disclosed in FIG. 1.
[0122]Varilrix™ is a commercially available OKA strain.
[0123]Human volunteers (for example, 50 per group—healthy, aged 50-70 years) can be selected to be vaccinated according to the above protocol, and results may be assessed by measuring both cell mediated immunity and antibody responses, for example by intracellular staining (ICS, Roederer et al. 2004 Clin. Immunol. 110: 199) or ELISA techniques respectively, these being well known in the art.
[0124]Specific cell-mediated immunity may be evaluated by, for example, in vitro incubation of patient PBMC with varicella-zoster virus extracts as well as specific VZV antigens or pep...
example 2
[0129]The experiment of Example 1 was carried out in human volunteers of different ages, as follows:
Group AgE AS1 in adults 18-30 yearsGroup BgE delivered concomitantly with the Varilrix OKA strain18-30 yearsGroup CVarilrix OKA strain alone in adults 50-70 yearsGroup DgE AS1 in adults 50-70 yearsGroup EgE delivered concomitantly with the Varilrix OKA strain50-70 years
[0130]The vaccination schedule was as follows:
GroupAge (Y)NVacc 1 (Month 0)Vacc 2 (Month 2)A18-3010gE-AS1gE-AS1B18-3010gE-AS1 + Varilrix ™gE AS1 + Varilrix ™C50-7045Varilrix ™Varilrix ™D50-7045gE-AS1gE-AS1E50-7045gE-AS1 + Varilrix ™gE-AS1 + Varilrix ™
[0131]The adjuvant AS1 comprises 3D MPL and QS21 in a quenched form with cholesterol, and was made as described in WO9633739, incorporated herein by reference. In particular the AS1 adjuvant was prepared essentially as Example 1.1 of WO9633739.
[0132]The adjuvant comprises: liposomes, which in turn comprise dioleoyl phosphatidylcholine (DOPC), cholesterol and 3D MPL [in an a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com