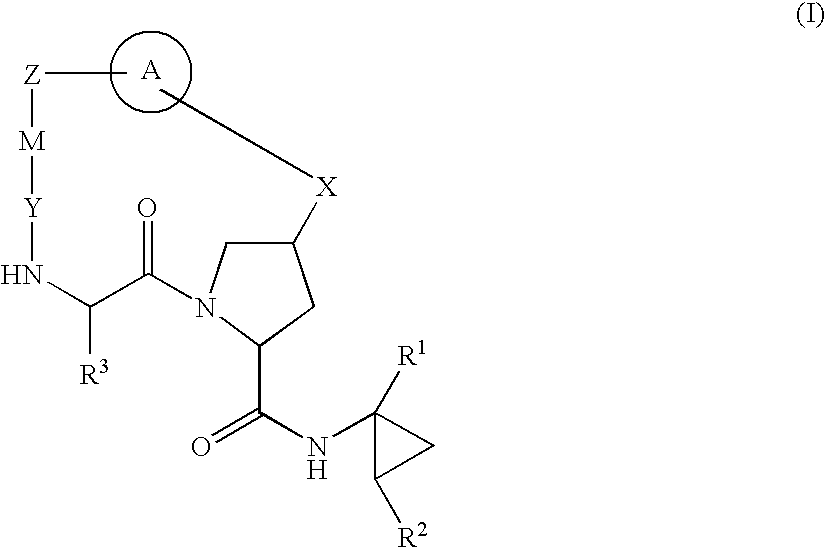
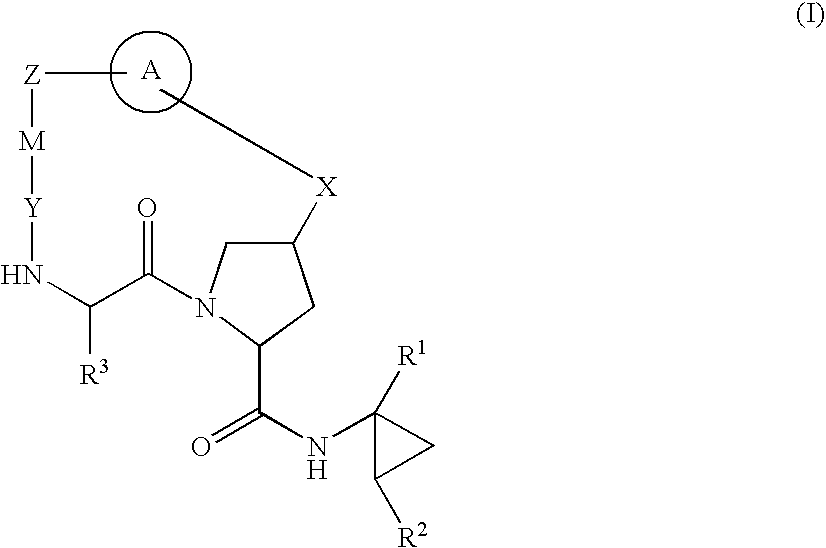
HCV NS3 Protease Inhibitors
a protease inhibitor and hcv technology, applied in the field of macrocyclic compounds, can solve the problems of limited clinical benefit, no established hcv vaccine, and treatment of hcv infection, and achieve the effect of reducing the likelihood or severity of one or more symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(2R,4S,7S,16E)-7-t-Butyl-N-((1R,2S)-1-{[(cyclopropylsulfonyl)amino]carbonyl}-2-vinylcyclopropyl)-6,9-dioxo-3,4,6,7,8,9,12,13,14,15-decahydro-2H,11H-2,5-methanopyrido[2,3-k][1,10,3,6]dioxadiazacyclononadecine-4-carboxamide
[0390]
Step 1: 1-t-Butyl 2-methyl(2S,4R)-4-[(3-bromopyridin-2-yl)oxy]pyrrolidine-1,2-dicarboxylate
[0391]
[0392]To a solution of 1-t-butyl 2-methyl(2S,4S)-4-{[(4-bromophenyl)sulfonyl]oxy}pyrrolidine-1,2-dicarboxylate (1.718 g, 3.70 mmol) and 3-bromopyridin-2-ol (0.773 g, 4.44 mmol) in NMP (18.50 mL) under N2, CS2CO3 (1.808 g, 5.55 mmol) was added. The mixture was then heated to 40° C. After 17 hours, the reaction was complete, and H2O and EtOAc were added. The organic layer was then extracted with H2O (3×), NaHCO3 (2×) and brine (2×). The organic layer was dried over MgSO4, and the solvent was removed in vacuo. The crude product was purified on SiO2 (gradient elution, 0-40% EtOAc / hexanes) to yield 1.04 g of the title compound. LRMS ESI+ ((M-Boc)+H)+ 301.2.
Step 2: 1-t-B...
example 2
(2R,4S,7S,16E)-7-t-Butyl-N-((1R,2R)-1-{[(cyclopropylsulfonyl)amino]carbonyl}-2-ethylcyclopropyl)-6,9-dioxo-3,4,6,7,8,9,12,13,14,15-decahydro-2H,11H-2,5-methanopyrido[2,3-k][1,10,3,6]dioxadiazacyclononadecine-4-carboxamide
[0404]
[0405]To a solution of a portion of the product from Example 1, Step 6 (90 mg, 0.196 mmol) in DMF (2.2 mL), Intermediate A3 (63 mg, 0.235 mmol), DIEA (0.137 mL, 0.78 mmol), and HATU (97 mg, 0.255 mmol) was added. After 1 hour, the mixture was partitioned between EtOAc and 1N HCl. The organic layer was separated, dried over MgSO4, and the solvent was removed in vacuo. The crude product was purified on SiO2 (gradient elution, 0-70% EtOAc / hexanes) to yield 110 mg of the title compound. LRMS ESI+ (M+H)+ 674.4.
example 3
[0406](2R,4S,7S)-7-t-Butyl-N-((1R,2S)-1-{[(cyclopropylsulfonyl)amino]carbonyl}-2-vinylcyclopropyl)-6,9-dioxo-3,4,6,7,8,9,12,13,14,15,16,17-dodecahydro-2H,11H-2,5-methanopyrido[2,3-k][1,10,3,6]dioxadiazacyclononadecine-4-carboxamide
Step 1: Methyl (2R,4S,7S)-7-t-butyl-6,9-dioxo-3,4,6,7,8,9,12,13,14,15,16,17-dodecahydro-2H,11H-2,5-methanopyrido[2,3-k][1,10,3,6]dioxadiazacyclononadecine-4-carboxylate
[0407]
[0408]To a solution of a portion of the product from Example 1, Step 5 (200 mg, 0.422 mmol) in EtOAc (4.5 mL), 10% Pd / C (22.5 mg, 0.021 mmol) was added. The mixture was then place under H2, stirred for 17 hours, and filtered through a pad of glass wool. The solvent was then removed in vacuo to yield 195 mg of the title compound. LRMS ESI+ (M+H)+ 476.4.
Step 2: (2R,4S,7S)-7-t-Butyl-6,9-dioxo-3,4,6,7,8,9,12,13,14,15,16,17-dodecahydro-2H,11H-2,5-methanopyrido[2,3-k][1,10,3,6]dioxadiazacyclononadecine-4-carboxylic acid
[0409]
[0410]To a solution of a portion of the product from Step 1 (190 mg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com