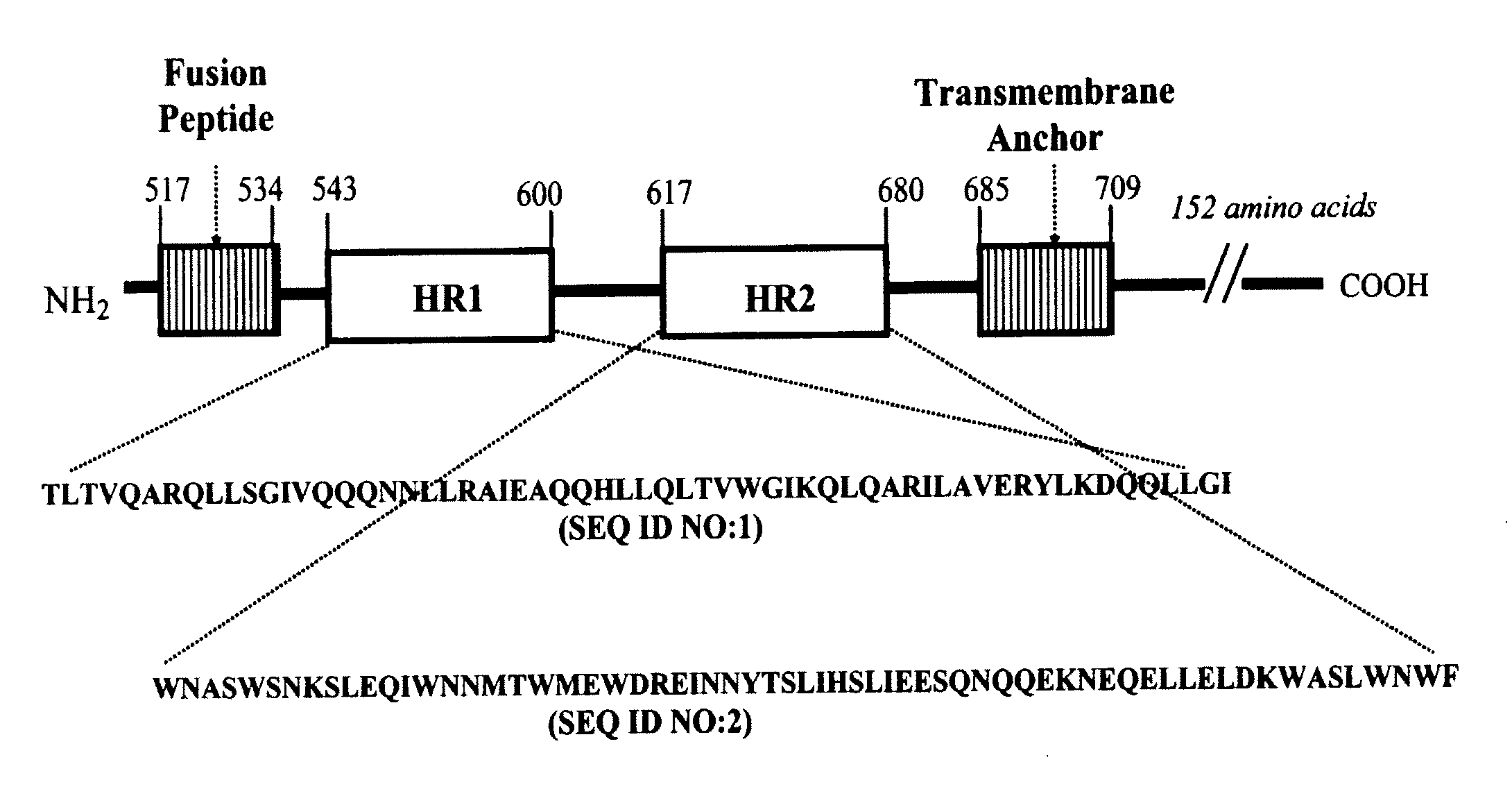
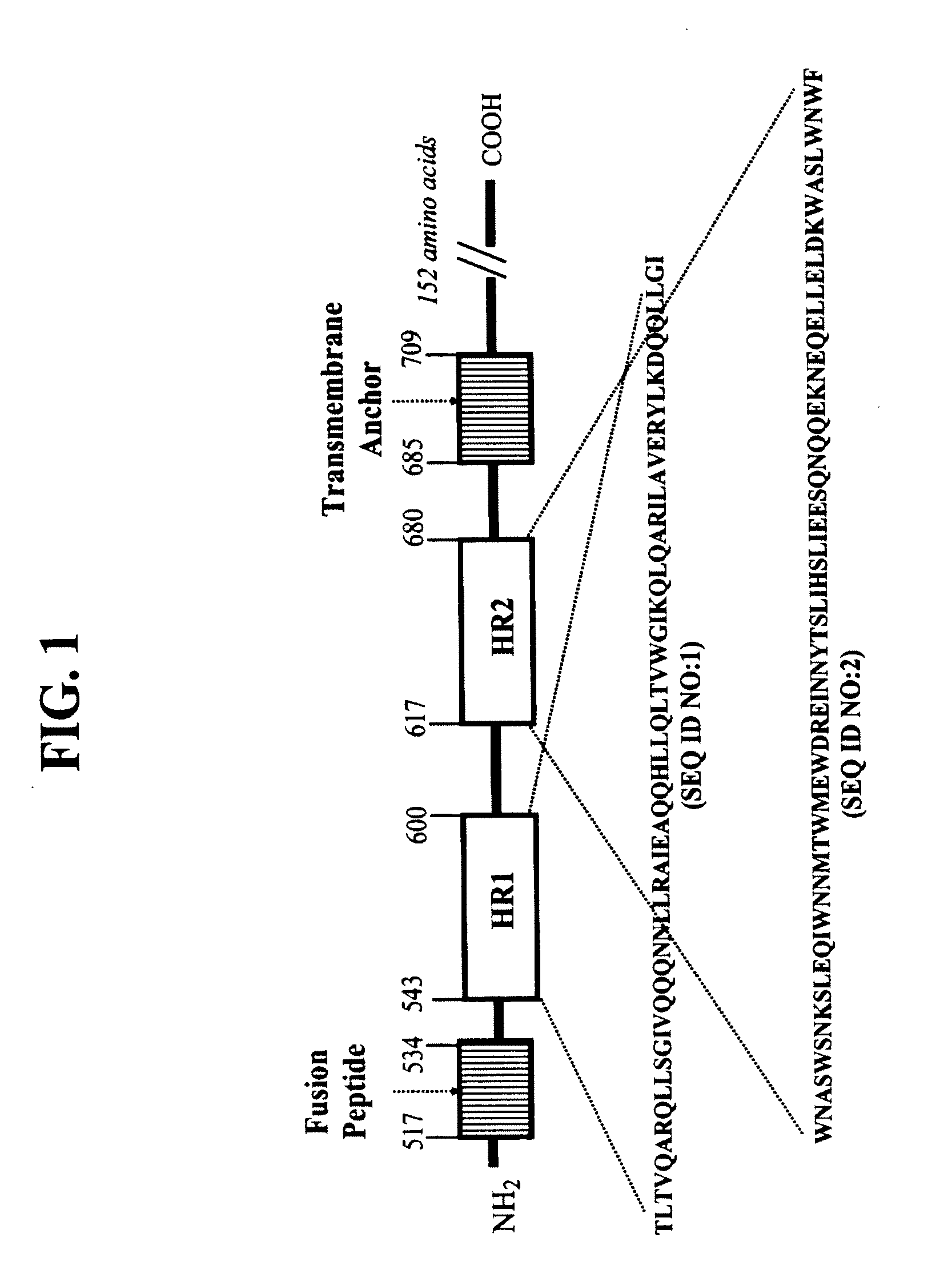
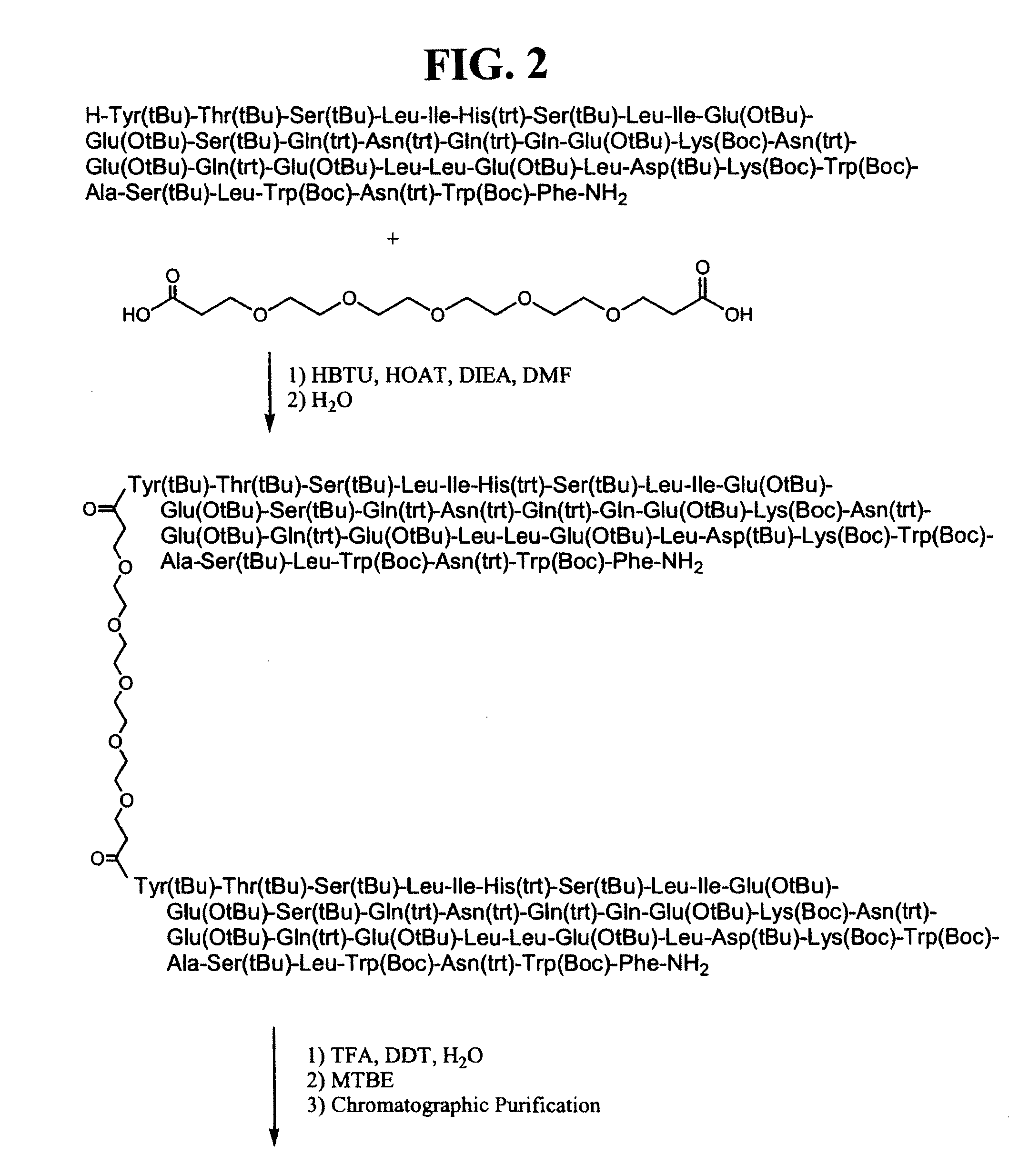
CONJUGATES COMPRISED OF POLYMER AND HIV gp-41-DERIVED PEPTIDES AND THEIR USE IN THERAPY
a technology of synthetic peptides and conjugates, which is applied in the field of conjugates comprised of polymer and synthetic peptides derived, can solve the problems of limiting the bioavailability of therapeutic agents, affecting the biological activity of therapeutic agents, so as to increase the biological half-life of synthetic peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0026]The embodiment illustrates a method of making the conjugates according to the present invention. One such method disclosed herein comprises the steps of: (a) reacting a first molecule of synthetic peptide with a polymer to form an intermediate comprising a first intermediate wherein the first molecule of synthetic peptide operably binds to a first reactive functionality of the polymer; (b) reacting the intermediate comprising a first intermediate with a second molecule of synthetic peptide to form a conjugate, wherein the second molecule of synthetic peptide operably binds to the intermediate comprising the first intermediate via a second reactive functionality of the polymer. It will be apparent to one skilled in the art that this method may also comprise adding a plurality of molecules of synthetic peptide simultaneously to a polymer, wherein more than one molecule of synthetic peptide becomes operably bound to the polymer in forming a conjugate, wherein each molecule of syn...
example 2
[0039]Illustrated in this example is the increased bioavailability (e.g., an extension of circulating half-life in vivo) of a conjugate according to the present invention as compared to the half-life of synthetic peptide alone. Using the methods and compositions taught in Example 1 herein, synthetic peptide and conjugate were produced. It is important to note that standard animal model for determining bioavailability has been correlated with the bioavailability of synthetic peptide in vivo in humans (as described in more detail in U.S. Pat. No. 6,258,782). Briefly, cannulated mice were dosed intravenously with either synthetic peptide, or a conjugate according to the present invention, with the dosing solution concentration being determined using the Edelhoch method, and as adjusted based on animal weight to achieve a 10 mg / kg dose. A sample of blood was removed at predetermined time intervals (0, 15, 30 min and 1, 2, 4, 6, and 8 hours) and added to collection tubes containing antic...
example 3
[0041]Illustrated in this example is the unexpected result of antiviral potency using the conjugates according to the present invention. In using an in vitro assay for demonstrating antiviral potency, it is important to note that antiviral effect of synthetic peptide demonstrated in the in vitro assay has been correlated with the antiviral effect of the synthetic peptide in vivo. In determining antiviral activity (e.g., one measure being the ability to inhibit transmission of HIV to a target cell) of the conjugates according to the present invention, used was an in vitro assay which has been shown, by data generated using synthetic peptides derived from either of the HR regions of HIV gp41, to be predictive of antiviral activity observed in vivo. More particularly, antiviral activity observed using an in vitro infectivity assay (“Magi-CCR5 infectivity assay”; see, e.g., U.S. Pat. No. 6,258,782) has been shown to reasonably correlate to antiviral activity observed in vivo for the sam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weights | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com