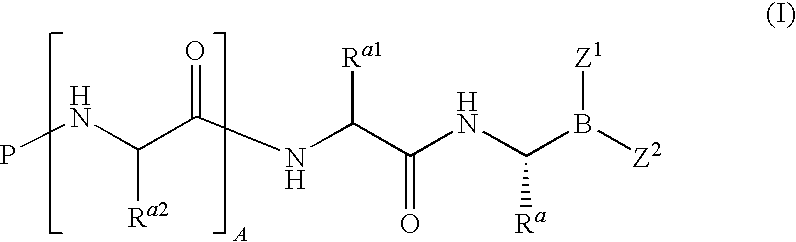
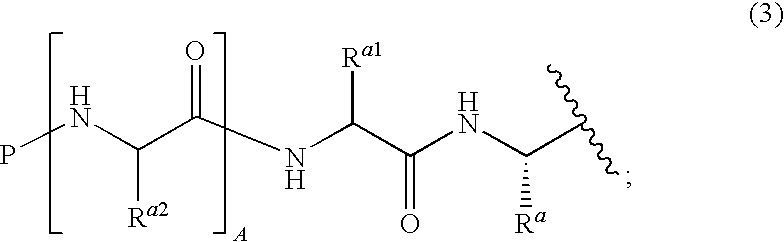
Boronate ester compounds and pharmaceutical compositions thereof
a technology of ester compounds and boroxines, which is applied in the field of boronate ester compounds, can solve the problems of limited pharmaceutical utility of boronic acid compounds, difficult to obtain analytically pure boronic acid compounds, and difficult to achieve alkylboronic acid and their boroxines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of 4-(R,S)-(carboxymethyl)-2-((R)-1-(2-(2,5-dichlorobenzamido)acetamido)-3-methylbutyl)-6-oxo-1,3,2-dioxaborinane-4-carboxylic Acid (I-1)
[0474]
Step 1: 2,5-[(dichlorobenzoyl)amino]acetic Acid
[0475]To a mixture of NaOH (12 g, 300 mmol) and glycine (18 g, 239 mmol) in water (120 mL) was added dropwise over 45 min a solution of 2,5-dichlorobenzoyl chloride (10 g, 48 mmol) in THF (15 mL) keeping the internal temperature below about 25° C. After 1 h, the mixture was acidified with 2.0 M HCl (125 mL) keeping the internal temperature below about 5° C. The resulting precipitate was collected by vacuum filtration. The crude product was recrystallized from water to give 2,5-[(dichlorobenzoyl)amino]acetic acid as a white, crystalline solid (6.1 g, 52%). mp 173.3° C. 1H NMR (300 MHz, DMSO-d6, δ): 12.72 (bs, 1H), 8.89 (t, J=6.0 Hz, 1H), 7.54 (m, 2H), 7.48 (m, 1H), 3.93 (d, J=6.0 Hz). 13C NMR (75 MHz, DMSO-d6, δ): 41.6, 129.3, 129.6, 131.4, 132.2, 138.2, 171.4, 165.9. MS (m / z): [M+H] cal...
example 1a
Alternate Synthesis of 4-(RS)-(carboxymethyl)-2-((R)-1-(2-(2,5-dichlorobenzamido)acetamido)-3-methylbutyl)-6-oxo-1,3,2-dioxaborinane-4-carboxylic Acid (I-1) Form 2
[0490]A 50-L glass reactor equipped with mechanical stirrer, dropping funnel, temperature indicator, and heating / cooling control unit (under nitrogen) was charged with 1.2 micron filtered EtOAc (18.9 kg) and anhydrous citric acid (0.561 kg, 2.9 mol). The mixture was heated to 71° C. and a solution resulted. N,N′,N″-{boroxin-2,4,6-triyltris[[(1R)-3-methylbutane-1,1-diyl]imino(2-oxoethane-2,1-diyl)]}tris(2,5-dichlorobenzamide) (1.109 kg, 3.1 mol) dissolved in EtOAc (4.0 kg) was clarified using an in-line filter (1.2 micron), and the solution was added to the reaction mixture under stirring (193 rpm) over a period of 20 min while maintaining a temperature of 73° C. to 75° C. The stirring was reduced to 96 rpm and the mixture was cooled as follows: (1) The mixture was kept at 73° C. 75° C. for 25 min; (2) The mixture was stepw...
example 2
Synthesis of 2,5-dichloro-N-(2-{[(1R)-3-methyl-1-(4-oxo-1,3,2-dioxaborolan-2-yl)butyl]amino}-2-oxoethyl)benzamide (I-2)
[0493]To a solution of glycolic acid (0.041 g, 0.54 mmol) in EtOAc (2.0 mL) with an internal temperature of about 60° C. was added a solution of N,N′,N″-{boroxin-2,4,6-triyltris[[(1R)-3-methylbutane-1,1-diyl]imino(2-oxoethane-2,1-diyl)]}tris(2,5-dichlorobenzamide) (0.199 g, 0.19 mmol) in EtOAc (1.0 mL). The solution was cooled uncontrolled until the internal temperature was about 25° C. and the solvent was removed by evaporation to yield 2,5-dichloro-N-(2-{[(1R)-3-methyl-1-(4-oxo-1,3,2-dioxaborolan-2-yl)butyl]amino}-2-oxoethyl)benzamide as a white solid (0.215 g, 95%). MS (m / z) in CH3CN: [M+Et3N+H] calculated for C22H35BCl2N3O5, 502.2; found, 502.0. MS (m / z) in CH3CN: [M−H] calculated for C16H18BCl2N2O5, 399.1; found, 399.0.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com