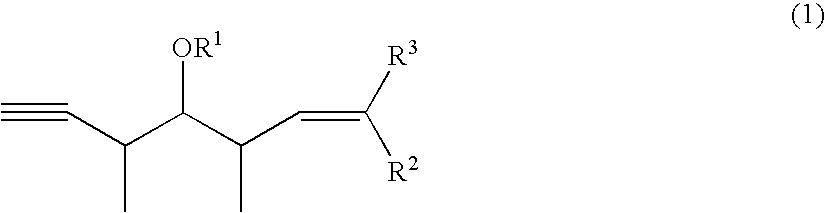
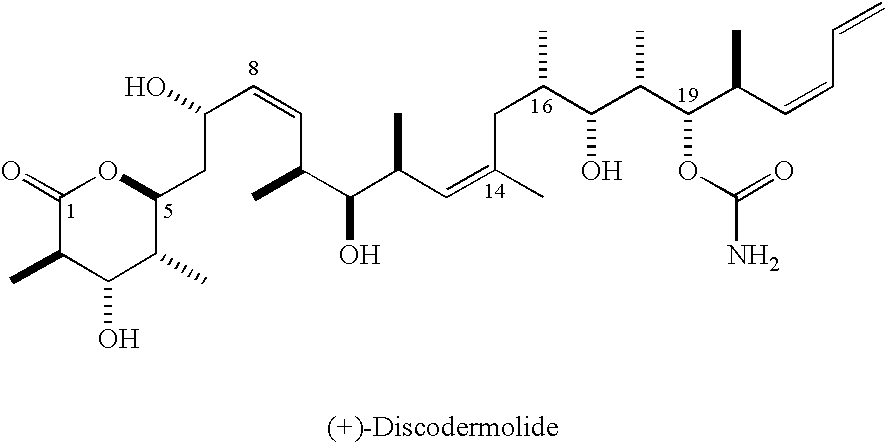
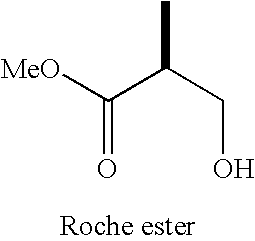
Intermediates for the synthesis of polypropionate antibiotics
a technology of intermediation and polypropionate antibiotics, applied in the field of discodermo, can solve the problems of commercially meaningful quantities of drugs and the formidable task of synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Racemic cis allylic alcohol (3)
[0055] A solution of cyclohexanecarboxaldehyde (2) (2.33 g, 20.8 mmol) in 40 mL of THF was cooled to −40° C. 1-Propynylmagnesium bromide (0.5 M in THF, 50.0 ML was added dropwise. After adding, the temperature of the reaction mixture was increased to 0° C. and stirred for 2 h. The reaction was quenched with saturated aqueous ammonium chloride solution. The water phase was extracted with ether (3×20 mL). The combined organic layers were washed with brine, dried over MgSO4, filtered, and concentrated. The crude product was chromatographed (HE:EA=10:1) to provide 2.78 g (88%) of the propargyl alcohol as an oil. 1H NMR (300 MHz, CDCl3) 4.10 (m, 1H), 1.85 (d, J=2.1 Hz, 3H), 1.80-1.65 (m, 5H), 1.58-1.20 (m, 1H), 1.30-0.98 (m, 6H); 13C NMR (300 MHz, CDCl3) 81.6, 79.5, 67.5, 44.4, 28.7, 28.2, 26.5, 26.02, 26.00, 3.7.
[0056] A solution of the propargyl alcohol from the above experiment (380 mg, 2.5 mmol) in hexane (3 mL) was treated with Pd / CaCO3 poisoned with...
example 2
Racemic Ether (4)
[0057] A 100-mL reaction flask was charged with 95% sodium hydride (2.76 g, 109 mmol) and 20 mL of dry THF. Racemic alcohol 3 (2.40 g, 15.6 mmol) in 2 mL of THF was added dropwise followed by 3-chloro-2-methylpropene (4.24 g, 46.8 mmol). The reaction mixture was stirred at reflux overnight and then cooled to room temperature. Excess sodium hydride was quenched by the slow addition of 3 mL of water. The resulting mixture was poured into water. Ether extracts (30 mL×3) were combined and the resulting organic solution was dried over MgSO4, filtered, and concentrated. Chromatography (HE:EA=30:1) produced a colorless oil (2.78 g, 85%). 1H NMR (300 MHz, CDCl3) 5.73 (dqd, J=10.2, 6.8, 1.3 Hz, 1H), 5.24 (ddq, 10.2, 9.4, 1.8 Hz, 1H), 4.93 (m, 1H), 4.85 (m, 1H), 3.90 (d, J=12.6 Hz, 1H), 3.80 (m, 1H), 3.69 (d, J=12.4 Hz, 1H), 1.96 (m, 1H), 1.73 (s, 3H), 1.70-1.65 (m, 5H ), 1.62 (dd, J=7.1, 2.0 Hz, 3H), 1.58-0.80 (m, 6H); 13C NMR (300 MHz, CDCl3) 143.3, 130.9, 128.1, 112.1, 77...
example 3
Racemic Dienol (5)
[0058] Potassium tert-butoxide (1.0 M in THF, 5.5 mL, 5.5 mmol) was added to a flask under argon and an additional 5.0 mL of THF was added. The solution was cooled to −78° C. and ether (4) (5.0 mmol, 1.05 g) was added. n-Butyllithium (1.6M in THF, 3.8 mL, 6.1 mmol) was slowly added. The mixture was warmed to −20° C. over 4 h and the stirred 12 h at −20° C. and 2 h at 0° C. The reaction was quenched with water and the resulting mixture was extracted with ether. The organic phase was dried over MgSO4 and concentrated. Chromatography (HE:EA=10:1) gave a colorless oil (819 mg, 78%). 1H NMR (300 MHz, CDCl3) 5.40 (dd, J=16.2, 6.6 Hz, 1H), 5.31 (dd, J=16.2, 6.6 Hz, 1H), 4.92 (m, 1H), 4.86 (m, 1H), 3.87 (d, J=20.0 Hz, 1H), 2.33 (m, 1H), 1.90 (m, 1H, 1.69 (s, 3H), 1.68-1.65 (m, 5H), 1.32-1.05 (m, 6H), 0.97 (d, J=6.9 Hz, 3H); 13C NMR (300 MHz, CDCl3) 146.2, 137.4, 129.9, 112.1, 79.3, 41.0, 40.1, 33.5, 33.4, 26.5, 26.4, 18.9, 14.8. IR 3403 (broad), 2965, 2924, 2851, 1448, 97...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com