Embolic protection devices
a technology of embolic protection and filter device, which is applied in the field of filter device and system, can solve the problems of emboli not being fully vaporized and thus entering the bloodstream, affecting the patient's health, and releasing emboli into the circulatory system can be extremely dangerous and sometimes fatal to the patient, so as to prevent accidental leakage of embolic debris
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
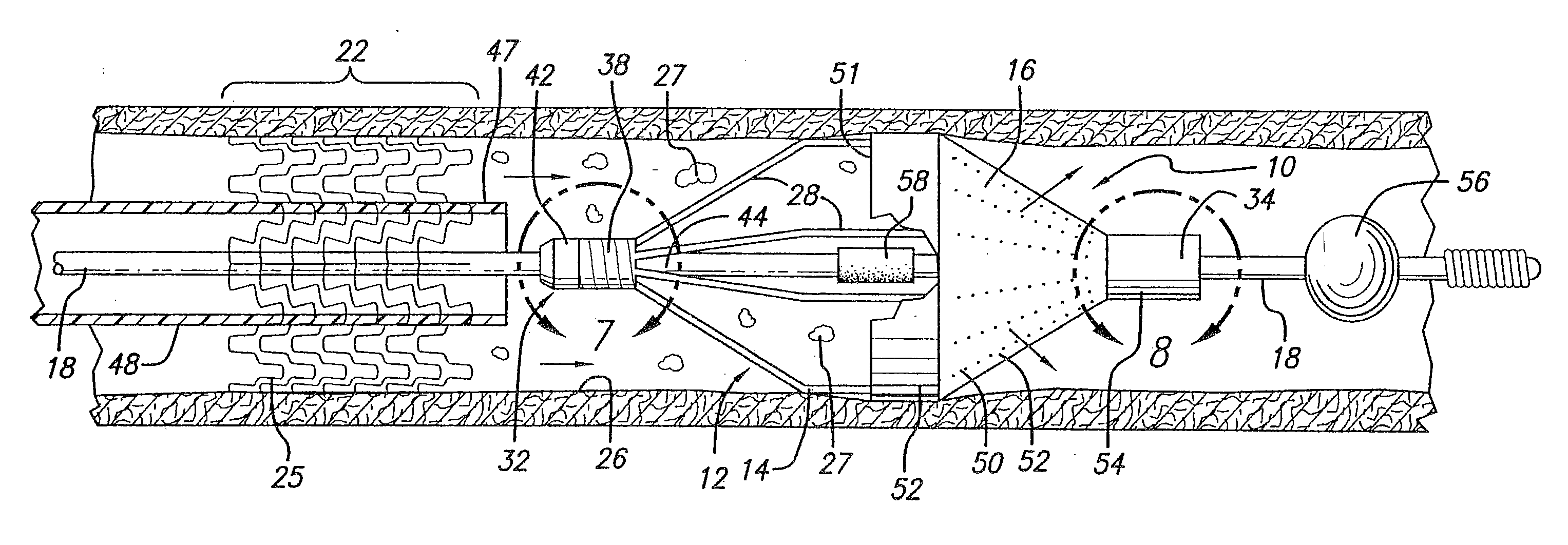
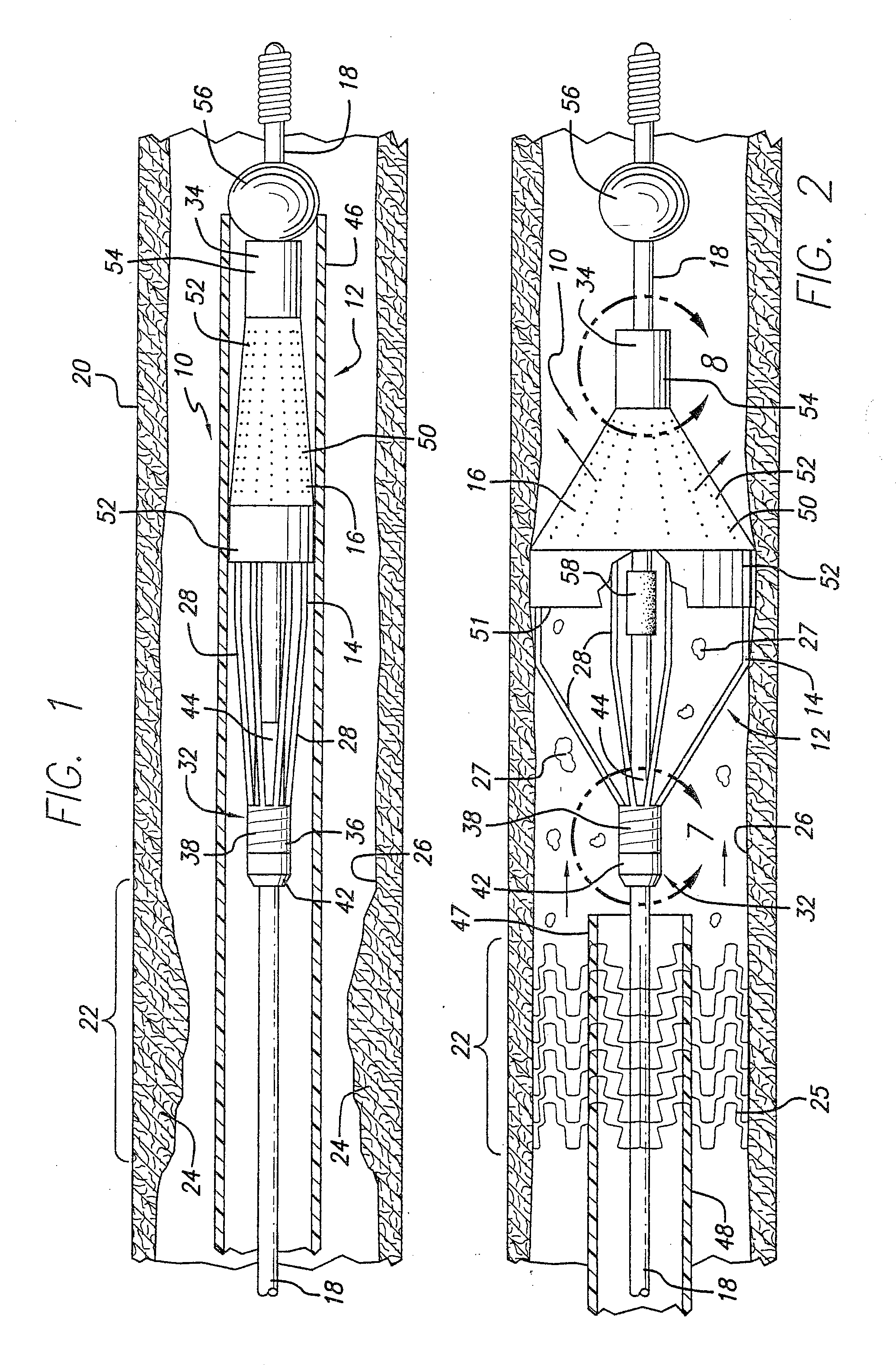
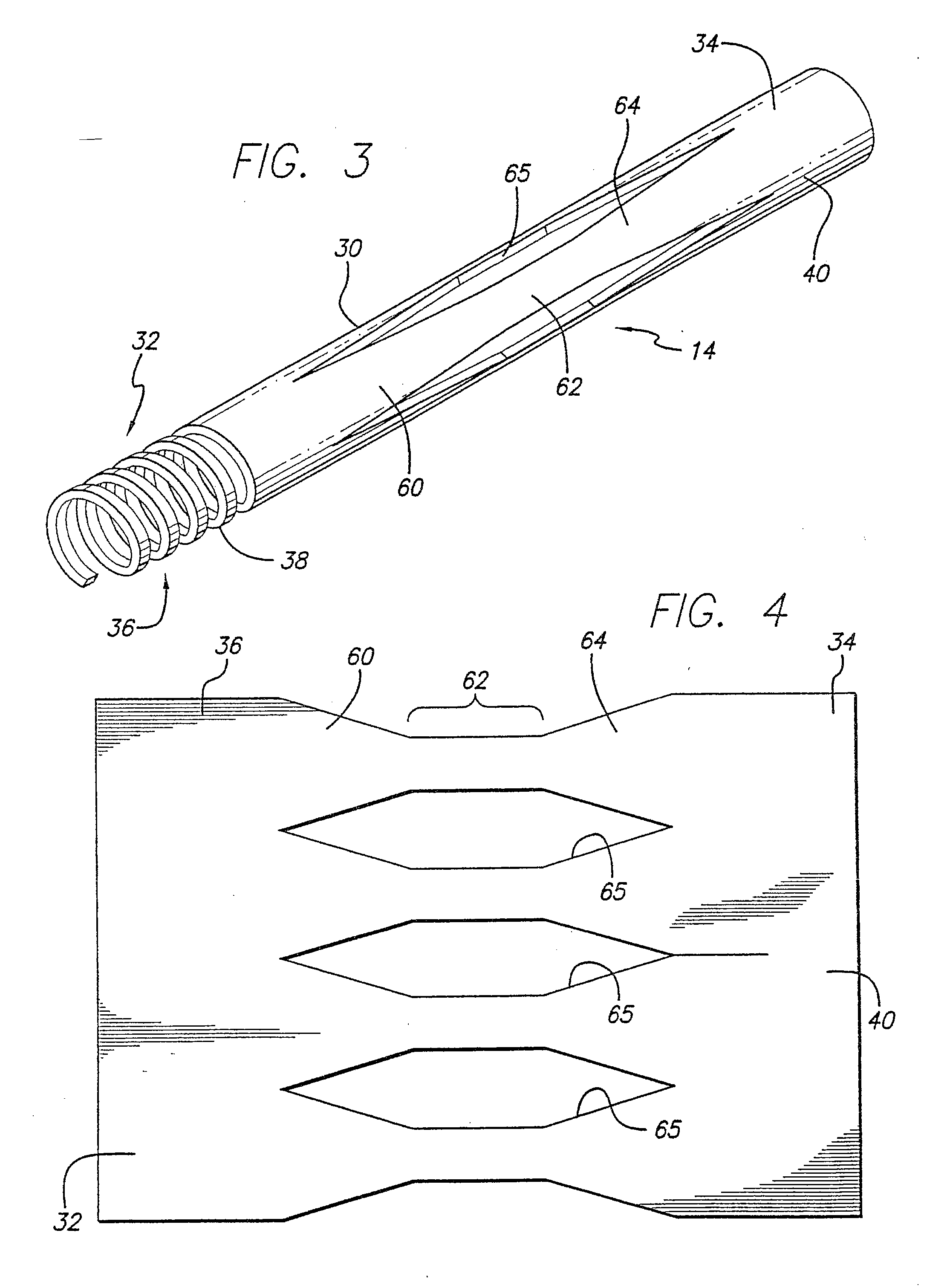
[0076] Turning now to the drawings, in which like reference numerals represent like or corresponding elements in the drawings, FIGS. 1 and 2 illustrate an embolic protection device 10 incorporating features of the present invention. In the particular embodiment shown in FIGS. 1 and 2, the embolic protection device 10 comprises a filter assembly 12 which includes an expandable strut assembly 14 and a filter element 16. The filter assembly 12 is rotatably mounted on the distal end of an elongated tubular shaft, such as a guide wire 18. Additional details regarding particular structure and shape of the various elements making up the filter assembly 12 are provided below.
[0077] The embolic protection device 10 is shown as it is placed within an artery 20 or other blood vessel of the patient. This portion of the artery 20 has an area of treatment 22 in which atherosclerotic plaque 24 has built up against the inside wall 26 of the artery 20. The filter assembly 12 is placed distal to, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| ultimate tensile strength | aaaaa | aaaaa |
| ultimate tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com