Sphincter treatment apparatus
a technology for sphincter and apparatus, which is applied in the direction of respirator, catheter, therapy, etc., can solve the problems of esophageal obstruction, significant blood loss and perforation of the esophagus, and more severe complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
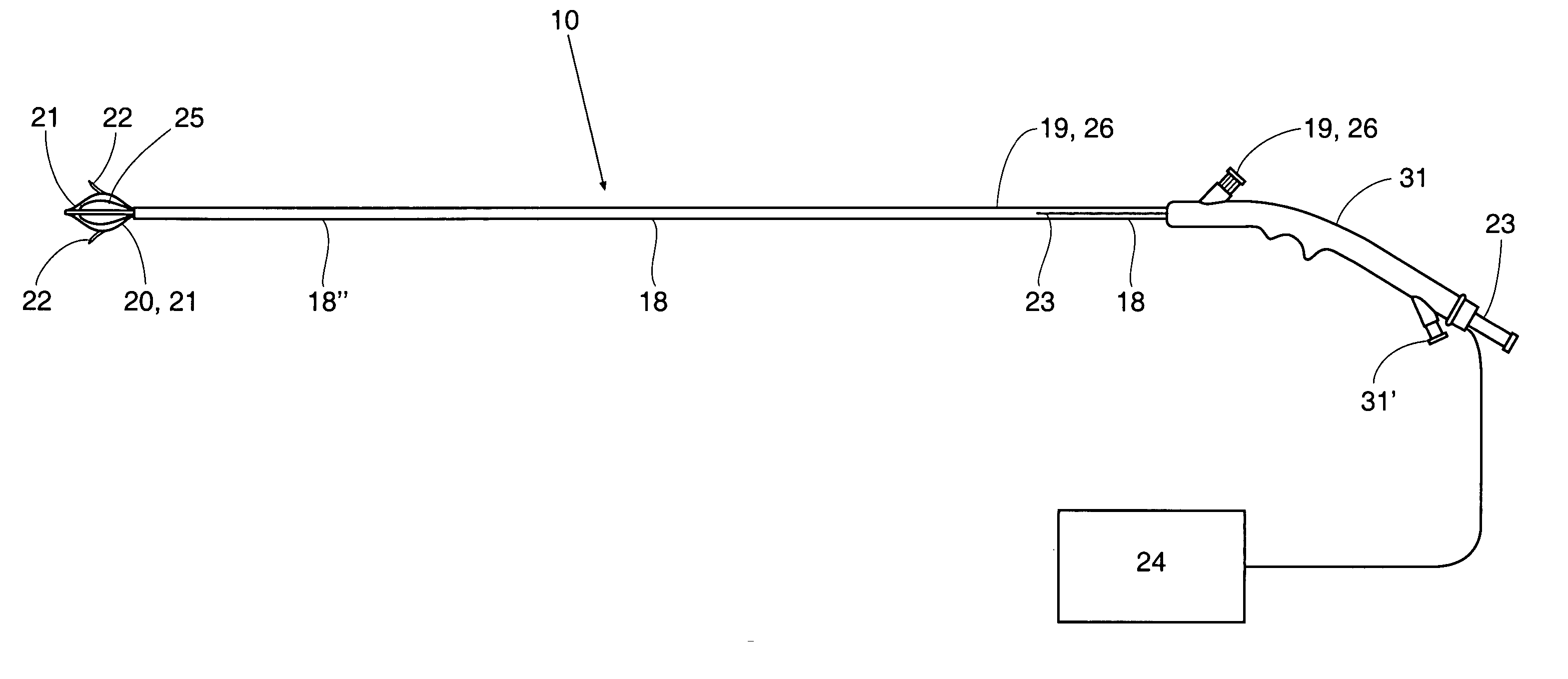
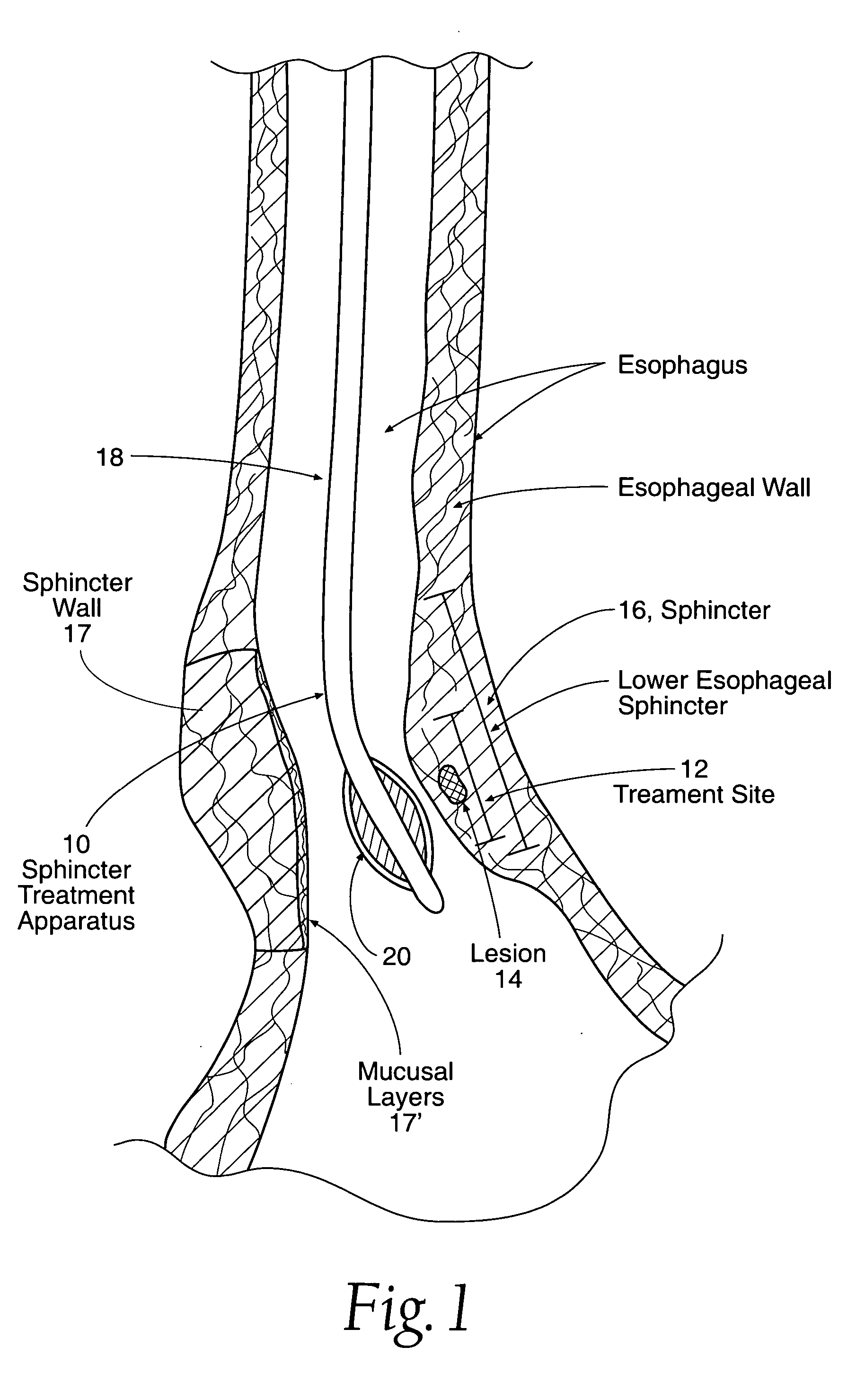
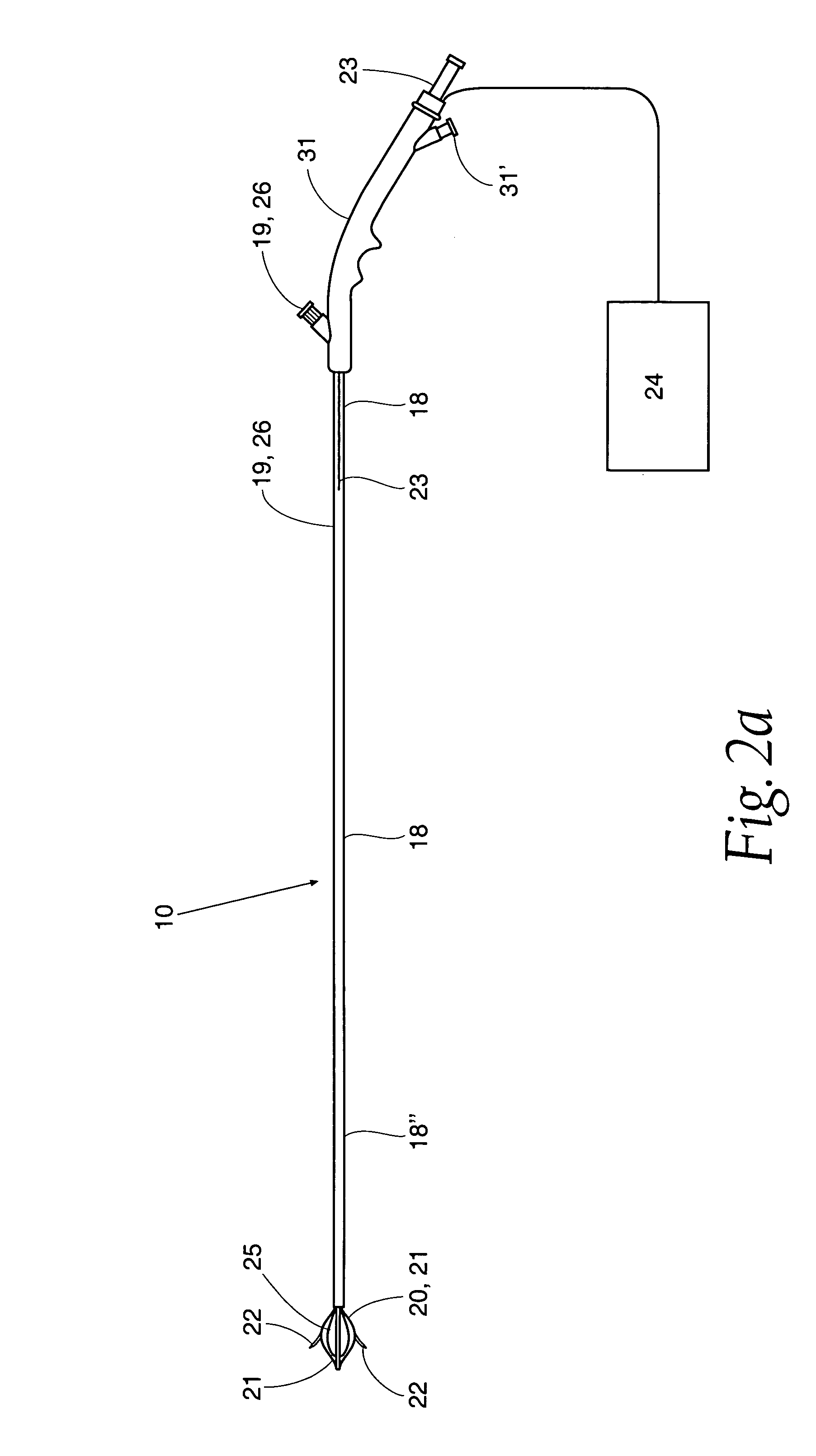
[0045] Referring now to FIG. 1, one embodiment of sphincter treatment apparatus 10 is illustrated. Apparatus 10 delivers energy to a treatment site 12 to produce lesions 14 in a sphincter 16, such as the lower esophageal sphincter (LES) having a wall 17 and mucosal layers 17′. Apparatus 10 includes a flexible elongate shaft 18 which can be an introducer, cannula, catheter and the like.
[0046] As illustrated in FIG. 2a, shaft 18 is coupled to a basket assembly 20. Basket assembly 20 is made of a plurality of arms 21. A plurality of energy delivery devices 22 are positioned and advanced from arms 21 into different circumferential regions of tissue site 12 or other treatment site within the sphincter wall 17 or adjacent anatomical structure. Energy delivery devices 22 are positioned, advanceable and retractable to and from basket assembly 20. Energy delivery devices 22 are positioned a desired depth in a sphincter wall 17 or adjoining anatomical structure. Energy delivery devices 22 ar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface areas | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com